1
Benzyne is a very reactive intermediate. As a result, it is very difficult to track chemical reactions. How was its existence demonstrated?
Choose one answer.
|
a. By trapping the intermediate in a cycloaddition reaction |
||
|
b. By separating it from the mixture by GC/MS |
||
|
c. By separating it from the mixture by column chromatography |
||
|
d. None of the above |
Question 2
Benzyne is the mechanistic intermediate for which of the following reactions?
Choose one answer.
|
a. Nucleophilic additions |
||
|
b. Electrophilic additions |
||
|
c. Cycloadditions |
||
|
d. All of the above |
Question 3
Chain reactions involving free-radicals occur in which of the following orders?
Choose one answer.
|
a. Initiation, propagation, termination |
||
|
b. Initiation, termination, propagation |
||
|
c. Propagation, termination, initiation |
||
|
d. Termination, propagation, initiation |
Question 4
Given that the energy of a CH3-H bond is 104 kcal/mol and that of an HO-H bond is 119 kcal/mol, what is the bond dissociation energy of the following reaction?
Choose one answer.
|
a. 15 kcal/mol |
||
|
b. -15 kcal/mol |
||
|
c. 223 kcal/mol |
||
|
d. -223 kcal/mol |
Question 5
Given that the energy of a CH3bond is 104 kcal/mol, CH3-Cl bond is 85 kcal/mol, H-Cl bond is 103 kcal/mol and that of an Cl2 bond is 58 kcal/mol, what is the bond dissociation energy of the following reaction?
Choose one answer.
|
a. 25 kcal/mol |
||
|
b. -25 kcal/mol |
||
|
c. 189 kcal/mol |
||
|
d. -166 kcal/mol |
Question 6
Identify the most stable free radical.
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 7
Nitrogen ylides are very versatile and can be used in a number of chemical reactions. Which of the following is a reaction of nitrogen ylides?
Choose one answer.
|
a. Cannizzaro reaction |
||
|
b. Sommelet reaction |
||
|
c. Blanc reaction |
||
|
d. Friedel-Crafts reaction |
Question 8
Nitrogen ylides are very versatile and can be used in a number of chemical reactions. Which of the following reactions is NOT a reaction of nitrogen ylides?
Choose one answer.
|
a. Stevens reaction |
||
|
b. Sommelet reaction |
||
|
c. Wittig reaction |
||
|
d. Friedel-Crafts reaction |
Question 9
The Wittig reagent was allowed to react with and carbonyl compound with the formula C10H12O. The name of the product was 4-phenyl-4-octene. What is the name of the carbonyl compound used?
Choose one answer.
|
a. 1-phenyl-1-butanone |
||
|
b. Acetophenone |
||
|
c. 2-phenyl-3-butanone |
||
|
d. Benzaldehyde |
Question 10
What are common methods for preparing benzyne?
Choose one answer.
|
a. Elimination from organolithium or organomagnesium precursors |
||
|
b. loss of neutral leaving group |
||
|
c. photolytic and/or pyrolitic methods |
||
|
d. All of the above |
Question 11
What is the major product of the radical reaction of 2-methylbutane with Cl2?
Choose one answer.
|
a. 2-chloro-2-methylbutane |
||
|
b. 1-chloro-2-methylbutane |
||
|
c. 3-chloro-2-methylbutane |
||
|
d. 4-chloro-2-methylbutane |
Question 12
Which halogen is most reactive in radical halogenations of alkanes?
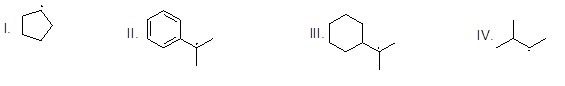
Choose one answer.
|
a. F2 |
||
|
b. Cl2 |
||
|
c. Br2 |
||
|
d. I2 |
Question 13
Which of the following is NOT a method for generating free radicals?
Choose one answer.
|
a. Thermal cracking |
||
|
b. Photolysis bond heterolysis |
||
|
c. Homolysis of peroxides and azo compounds |
||
|
d. Electron transfer |
Question 14
Which of the following is NOT a precursor of Benzyne?
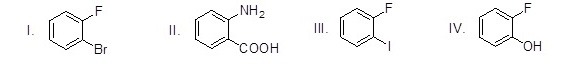
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 15
Which of the following molecules is a nitrogen ylide?
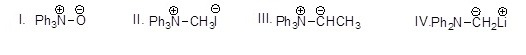
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 16
Which of the following molecules is a phosphorus ylide?
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 17
Which of the following molecules is a sulfur ylide?
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 18
Which of the following six-carbon molecules will form a single chlorinated compound when reacting with chlorine in presence of light.
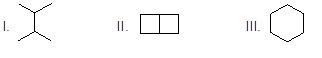
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. All of them |
Question 19
Which of the following Wittig reagents would be useful for converting acetophenone into 1,1-diphenylethene?
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 20
Which statement about free radicals is incorrect?
Choose one answer.
|
a. Free radicals contain an unpaired electron. |
||
|
b. Radicals are believed to be involved in degenerative diseases and cancers. |
||
|
c. They are formed by heterolytic cleavage of bonds. |
||
|
d. Radicals can either be neutral or ionic. |
Question 21
1-chloro-3-methylhexane undergoes SN2 reaction with sodium ethoxide. This reaction yields low amount of products. However, addition of small amounts of sodium iodide increases the rate of the reaction. Why?
Choose one answer.
|
a. The charge of the sodium ion stabilizes the charge of the chloride ion. |
||
|
b. The iodide ion renders the ethoxide ion more reactive. |
||
|
c. Small amounts of alkyl chlorides are converted into the more reactive alkyl iodides. |
||
|
d. With the presence of NaI, the mechanism changes to Sn1. |
Question 22
Compound A undergoes a rearrangement reaction and forms compounds B and C. Based on the potential energy diagram below, what can be said about compounds B and C in terms of kinetics and thermodynamics?
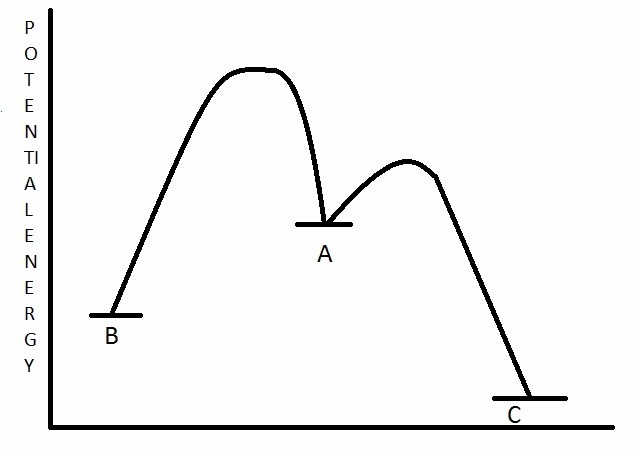
Choose one answer.
|
a. B is the kinetic and thermodynamic product. |
||
|
b. B is the kinetic product, and C is the thermodynamic product. |
||
|
c. C is the kinetic product, and B is the thermodynamic product. |
||
|
d. C is the kinetic and thermodynamic product. |
Question 23
Provide the IUPAC name, including stereochemical designation, for the following molecule.
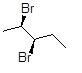
Choose one answer.
|
a. (2R,3R)-2,3-dibromopentane |
||
|
b. (2S,3S)-2,3-dibromopentane |
||
|
c. (2R,3S)-2,3-dibromopentane |
||
|
d. (2S,3R)-2,3-dibromopentane |
Question 24
Select the mechanism by which the reactants below will react.
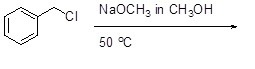
Choose one answer.
|
a. SN1 |
||
|
b. SN2 |
||
|
c. E1 |
||
|
d. E2 |
Question 25
Vinyl chloride, shown below, does not react with sodium hydroxide in SN2 reactions. Why?
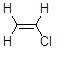
Choose one answer.
|
a. Hydroxide is a weak nucleophile. |
||
|
b. The sp2 carbon-chlorine bond is stronger than the sp3 carbon-chlorine bond. |
||
|
c. The chlorine atom is sterically crowded. |
||
|
d. Both B and C |
Question 26
What is the configuration at the carbons labeled 1 and 2 in the following compound?
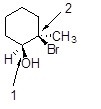
Choose one answer.
|
a. 1R, 2S |
||
|
b. 1S, 2R |
||
|
c. 1R, 2R |
||
|
d. 1S, 2S |
Question 27
What is the major product of the following reaction?
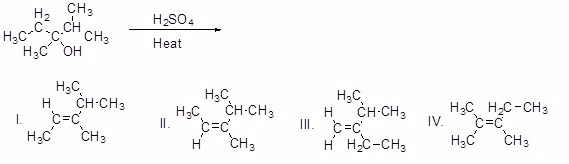
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 28
What is the relationship between the two compounds below?
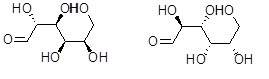
Choose one answer.
|
a. Same compound |
||
|
b. Structural isomers |
||
|
c. Enantiomers |
||
|
d. Diastereomers |
Question 29
What reaction will the following compound most likely undergo?
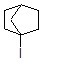
Choose one answer.
|
a. SN1 |
||
|
b. SN2 |
||
|
c. E1 |
||
|
d. E2 |
Question 30
Which is the correct structural formula for the compound formed according to the mechanism below (lone electron pairs were omitted)?
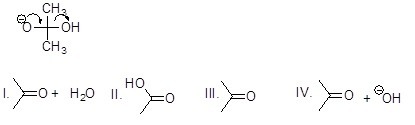
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 31
Which of the following alkyl halides give only one alkene as the product in the E2 reaction?
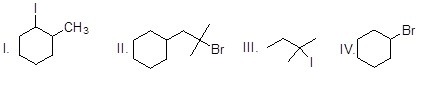
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 32
Which of the following carbocations is most stable?
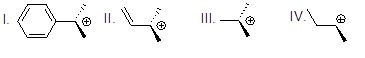
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 33
Which of the following compound h as the IUPAC name of (2R, 3R)-2, 3-dihydroxybutane?

Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 34
Which of the following compounds is chiral?
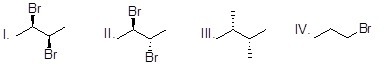
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 35
Which of the following compounds will be the most reactive substrate in SN1 reactions?
Choose one answer.
|
a. (C2H5)3CCl |
||
|
b. (C2H5)2CHCl |
||
|
c. C2H5CH2Cl |
||
|
d. (C2H5)3CF |
Question 36
Which of the following compounds will most readily react in S¬N1 reactions?
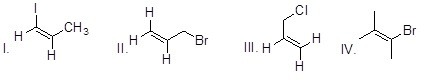
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 37
Which of the following molecules is prochiral?
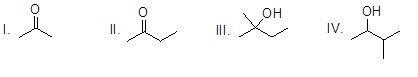
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 38
Which of the following statements about chirality is incorrect?
Choose one answer.
|
a. A chiral center is an atom that is tetrahedrally bonded to four different groups. |
||
|
b. A chiral molecule has a superimposable mirror image. |
||
|
c. A chiral molecule does not have any reflective symmetry. |
||
|
d. All chiral molecules may exist as enantiomers. |
Question 39
Which of the following statements about the chemical reaction described below is true?
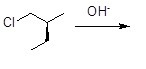
Choose one answer.
|
a. The product will have R configuration. |
||
|
b. The product will have S configuration. |
||
|
c. The product will be achiral. |
||
|
d. The product will be a racemic mixture. |
Question 40
Which of the following statements best describes an E2 reaction?
Choose one answer.
|
a. It involves a two-step mechanism. |
||
|
b. It involves a very reactive intermediate. |
||
|
c. The rate of the reaction depends on the concentration of the base. |
||
|
d. The rate of the reaction increases in polar solvents. |
Question 41
Name the following compound.
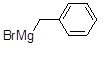
Choose one answer.
|
a. Phenylmagnesium bromide |
||
|
b. Ethylphenylmagnesium bromide |
||
|
c. Benzylmagnesium bromide |
||
|
d. Benzylicmagnesium bromide |
Question 42
The Grignard reaction is an important reaction to provide which of the following?
Choose one answer.
|
a. Carbon-carbon bonds |
||
|
b. Carbon-magnesium bonds |
||
|
c. Carbon-halide bonds |
||
|
d. Magnesium-halide bonds |
Question 43
What happens to the chiral center when (R)-3-methyl-3-phenyl-4-heptanone reacts with phenylmagnesium bromide?
Choose one answer.
|
a. Inversion of configuration occurs. |
||
|
b. A racemic mixture is formed. |
||
|
c. Nothing, the chiral center remains unchanged. |
||
|
d. The product is achiral. |
Question 44
What is organometallic chemistry?
Choose one answer.
|
a. The study of carbon containing compounds |
||
|
b. The study of compounds containing a carbon-metal bond |
||
|
c. The study of metals |
||
|
d. The study of salts |
Question 45
What is the chemical structure of furan?
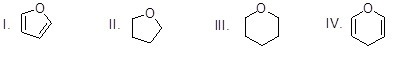
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 46
What is the IUPAC name for the imidazole molecule, shown below?
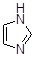
Choose one answer.
|
a. Imidazole |
||
|
b. 1,3-imidazole |
||
|
c. Diazole |
||
|
d. 1,3-diazole |
Question 47
What is the major product of the following reaction?
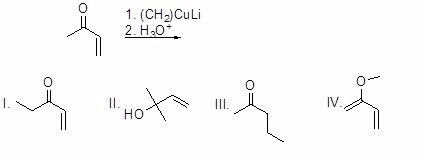
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 48
What is the major product of the following reaction?
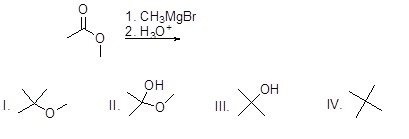
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 49
What is the name of the following heterocycle?
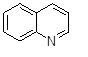
Choose one answer.
|
a. Isoquinoline |
||
|
b. Indole |
||
|
c. Quinoline |
||
|
d. Pyrrolidine |
Question 50
What is the name of the following heterocycle?
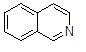
Choose one answer.
|
a. Isoquinoline |
||
|
b. Indole |
||
|
c. Quinoline |
||
|
d. Pyrrolidine |
Question 51
What is the name of the following heterocycle?
Choose one answer.
|
a. Thiazole |
||
|
b. Indole |
||
|
c. Quinoline |
||
|
d. Pyrrolidine |
Question 52
Which of the following alkyl halides would be suitable to use when forming a Grignard reagent?
Choose one answer.
|
a. CH3CH2CH(OH)CH2Br |
||
|
b. BrCH2CO2H |
||
|
c. PhCH2CH2Br |
||
|
d. None of these are suitable |
Question 53
Which of the following heterocycles is most basic?
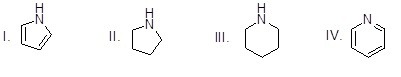
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 54
Which of the following is an organometallic compound?
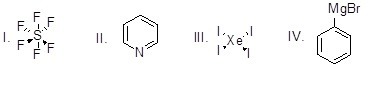
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 55
Which of the following molecules is an heterocycle?
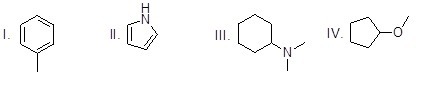
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 56
Which of the following molecules will NOT be a product of the following reaction?
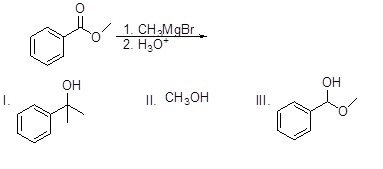
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. All of the above |
Question 57
Which of the following reactions would be a useful way of preparing 1-phenyl-1-butanol?
Choose one answer.
|
a. Butanal and phenylmagnesium bromide |
||
|
b. 1-phenyl-3-butanone and NaBH4 |
||
|
c. Propanal and benzylmagnesium bromide |
||
|
d. Both A and C |
Question 58
Which of the heterocyclic compounds shown below is most acidic?
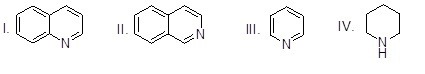
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 59
Which of the heterocyclic compounds shown below is most basic?
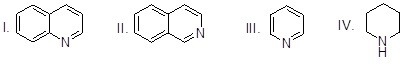
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. IV |
Question 60
Which reagent is best for the reaction below?
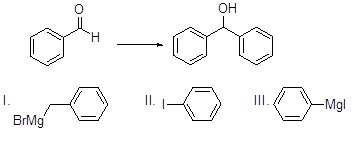
Choose one answer.
|
a. I |
||
|
b. II |
||
|
c. III |
||
|
d. None of the above |