1
What is an intensive property?
Choose one answer.
|
a. A house |
||
|
b. A physical property of a system that is independent of the system size and mass |
||
|
c. An extensive property |
||
|
d. A product of two extensive properties |
Question 2
What is a BTU?
Choose one answer.
|
a. A traditional unit of energy equal to 1055 J |
||
|
b. A traditional unit of energy equal to 1055 N |
||
|
c. A unit of energy that stand for British Transition Unit |
||
|
d. A unit of energy that stands for British Thermal Union |
Question 3
Which of the following statements about a closed system is true?
Choose one answer.
|
a. In a closed system, no mass may be transferred in or out of the system boundaries. |
||
|
b. In a closed system, no energy may be transferred in or out of the system boundaries. |
||
|
c. In a closed system, both mass and energy cannot be transferred in or out of the system boundaries. |
||
|
d. In a closed system, no work may be transferred in or out of the system boundaries. |
Question 4
What is an extensive property?
Choose one answer.
|
a. A specific property |
||
|
b. A physical property of a system that depends on the system size and mass |
||
|
c. An intensive property |
||
|
d. A product of two intensive properties |
Question 5
On a day when the temperature is 70°F, what is the temperature in degrees Kelvin?
Choose one answer.
|
a. 203°K |
||
|
b. 294°K |
||
|
c. 270°K |
||
|
d. 343°K |
Question 6
The boiling temperature of a substance is 500°F, what is the temperature in degrees Kelvin?
Choose one answer.
|
a. 303°K |
||
|
b. 260°K |
||
|
c. 533°K |
||
|
d. 500°K |
Question 7
What is a thermodynamic cycle?
Choose one answer.
|
a. A series of identical thermodynamic processes |
||
|
b. A thermodynamic process |
||
|
c. A system in equilibrium |
||
|
d. A series of thermodynamic processes that return to the first state of the first process |
Question 8
If two systems are at thermal equilibrium, which of the following properties of these systems are the same?
Choose one answer.
|
a. Internal energy |
||
|
b. Temperature |
||
|
c. Heat capacity |
||
|
d. Entropy |
Question 9
If the units of a are inch/lb (mass) and the units of b are kg2/m2, what are the units of a.b?
Choose one answer.
|
a. kg/m |
||
|
b. inch/lb |
||
|
c. kg |
||
|
d. m |
Question 10
Which of the following is NOT an equation of state?
Choose one answer.
|
a. Boyle's law |
||
|
b. Ideal gas law |
||
|
c. Newton’s second law |
||
|
d. f(P,V,T) = 0 |
Question 11
A rigid vessel is maintained at 10 MPa and 300°C. It also contains 800 g of steam. What is the volume of the vessel?
Choose one answer.
|
a. 1.5 m3 |
||
|
b. 1.1 m3 |
||
|
c. 0.1 m3 |
||
|
d. 1.9 m3 |
Question 12
A system contains water at 4 MPa and 300°C. What is the phase of this water?
Choose one answer.
|
a. Vapor |
||
|
b. Liquid-vapor mixture |
||
|
c. Liquid |
||
|
d. Solid |
Question 13
A system contains water at 4 MPa and 200°C. What is the phase of this water?
Choose one answer.
|
a. Vapor |
||
|
b. Liquid-vapor mixture |
||
|
c. Liquid |
||
|
d. Solid |
Question 14
What is the mass flow rate of a water-steam at 1 MPa and 300°C following through a 5 cm diameter pipe with an average velocity of 20 m/s?
Choose one answer.
|
a. 0.52 kg/s |
||
|
b. 0.152 kg/s |
||
|
c. 0.258 kg/s |
||
|
d. 1.65 kg/s |
Question 15
What is the boiling temperature of water at 4 MPa? (Hint: Use water-steam phase diagram shown here.)
Terms of Use: This image is licensed under the terms of the GNU Free Documentation License, Version 1.2 or any later version published by the Free Software Foundation; with no Invariant Sections, no Front-Cover Texts, and no Back-Cover Texts. It is also licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license. It is attributed to Markus Schweiss. The original version can be found here.
Terms of Use: This image is licensed under the terms of the GNU Free Documentation License, Version 1.2 or any later version published by the Free Software Foundation; with no Invariant Sections, no Front-Cover Texts, and no Back-Cover Texts. It is also licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license. It is attributed to Markus Schweiss. The original version can be found here.
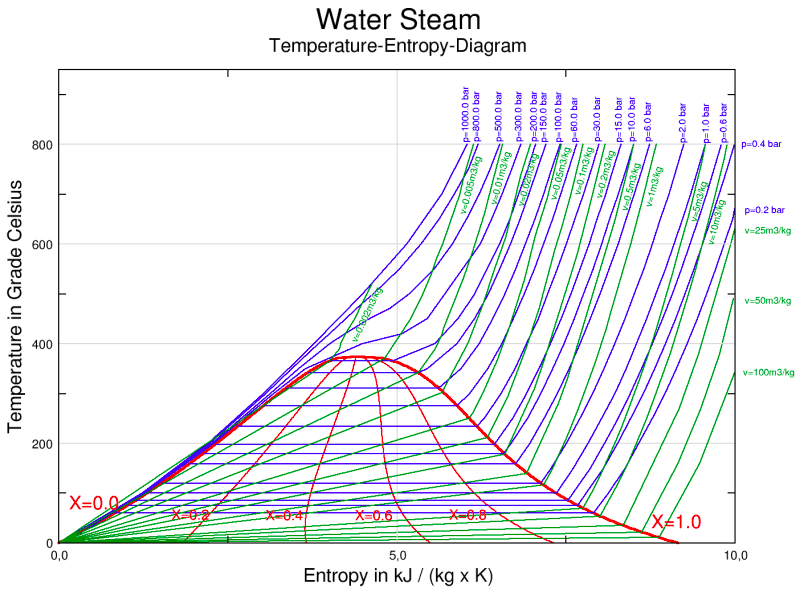
Choose one answer.
|
a. 280°C |
||
|
b. 100°C |
||
|
c. 300°C |
||
|
d. 250°C |
Question 16
A rigid vessel contains 7 kg of an ideal gas at 5 atm. A valve is opened, and half of the mass of the gas escapes. If the final pressure in the tank is 1.5 atm and the final temperature in the tank is -91°C, what is the initial temperature of the gas?
Choose one answer.
|
a. 300°C |
||
|
b. 30°K |
||
|
c. 30°C |
||
|
d. 300°K |
Question 17
A rigid vessel contains water vapor mixture in equilibrium at a pressure of 4.76 MPa. What is the temperature of the system?
Choose one answer.
|
a. 150°C |
||
|
b. 200°K |
||
|
c. 202°C |
||
|
d. 100°K |
Question 18
In a system, a water vapor mixture is at equilibrium at 200°C. What is the pressure of the system?
Choose one answer.
|
a. 3.25 MPa |
||
|
b. 4.76 MPa |
||
|
c. 1.76 bar |
||
|
d. 15 atm |
Question 19
A vessel contains an ideal gas at temperature T. What is the temperature of the gas if its volume is doubled and its pressure remains the same?
Choose one answer.
|
a. 2 T |
||
|
b. 1.5 T |
||
|
c. 0.6 T |
||
|
d. 0.5 T |
Question 20
What are temperature and pressure of water at the triple point?
Choose one answer.
|
a. 100°C and 1 atm |
||
|
b. 0°K and 611 Pa |
||
|
c. 273°K and 1 MPa |
||
|
d. 273°K and 611 Pa |
Question 21
What is an adiabatic process?
Choose one answer.
|
a. A process in which the system is perfectly insulated and heat transfer is zero |
||
|
b. A process in which the temperature stays constant |
||
|
c. A process in which the pressure stays constant |
||
|
d. A process in which the volume of the system stays constant |
Question 22
How much work is required to accelerate a mass of 10 kg from 10 km/hr to 100 km/hr?
Choose one answer.
|
a. 29.5 kJ |
||
|
b. 19.5 kJ |
||
|
c. 49.5 kJ |
||
|
d. 89.5 kJ |
Question 23
Which of the following is NOT a type of energy?
Choose one answer.
|
a. Heat |
||
|
b. Work |
||
|
c. Potential energy |
||
|
d. Entropy |
Question 24
What is heat conduction?
Choose one answer.
|
a. The transfer of thermal energy between regions of matter due to a temperature gradient |
||
|
b. The transfer of energy due to bulk movement of liquids |
||
|
c. The transfer of electrical energy from one object to another |
||
|
d. The transfer of kinetic energy due to collision |
Question 25
What are three major modes of heat transfer?
Choose one answer.
|
a. Radiation, friction, and convection |
||
|
b. Convection, isobaric, and radiation |
||
|
c. Conduction, isothermal, and isentropic |
||
|
d. Conduction, convection, and radiation |
Question 26
What temperature change on the Celsius scale is equivalent to a 50 degree change on the Kelvin scale?
Choose one answer.
|
a. 70°C |
||
|
b. 50°C |
||
|
c. 30°C |
||
|
d. 20°C |
Question 27
A 0.05 kg of metal at 446 K is dropped into a water tank that contains 0.94 lbs water initially at 23°C. What is the final temperature of the mixed system, if the specific heat of the metal is 574 J/kg C and the specific heat of water is 4186 J/kg C?
Choose one answer.
|
a. 35.9°C |
||
|
b. 75.2°C |
||
|
c. 125.6°C |
||
|
d. 25.4°C |
Question 28
A 200-m3 rigid vessel contains a saturated liquid-vapor mixture with a vapor quality of 75%. The temperature of the vessel is maintained at 393°K. What is the total mass of the liquid-vapor mixture inside the vessel?
Choose one answer.
|
a. 219 kg |
||
|
b. 299 kg |
||
|
c. 179 kg |
||
|
d. 359 kg |
Question 29
A rigid vessel contains 5 m3 of nitrogen (N2) at 252 °K and 10 MPa. What is the mass of nitrogen? (Hint: Use data from the compressibility chart for nitrogen shown here.)
Terms of Use: The image below is licensed under the terms of the GNU Free Documentation License, Version 1.2 or any later version published by the Free Software Foundation; with no Invariant Sections, no Front-Cover Texts, and no Back-Cover Texts. It is also licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license. It is attributed to Wikimedia user Aushulz. The original version can be found here.
Terms of Use: The image below is licensed under the terms of the GNU Free Documentation License, Version 1.2 or any later version published by the Free Software Foundation; with no Invariant Sections, no Front-Cover Texts, and no Back-Cover Texts. It is also licensed under the Creative Commons Attribution-Share Alike 3.0 Unported license. It is attributed to Wikimedia user Aushulz. The original version can be found here.
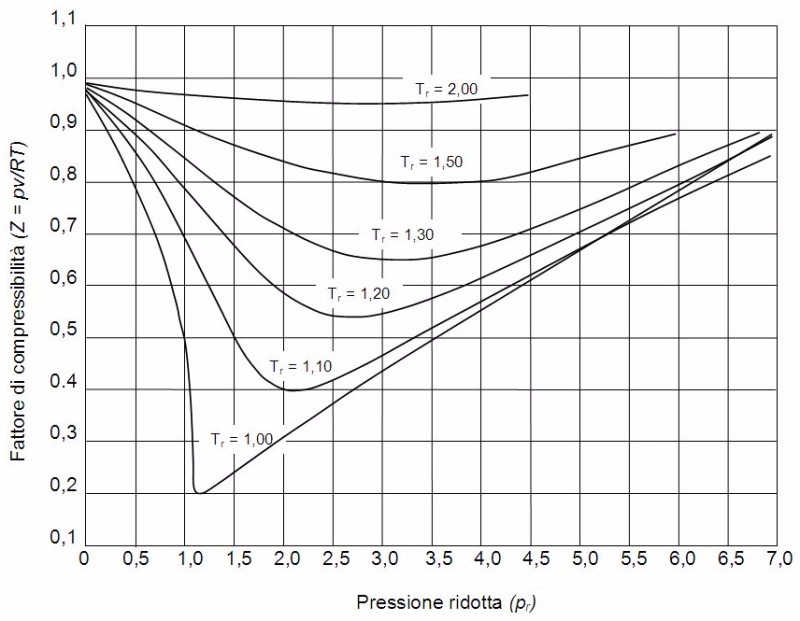
Choose one answer.
|
a. 1223 kg |
||
|
b. 142 kg |
||
|
c. 697 kg |
||
|
d. 47 kg |
Question 30
According to the first law of thermodynamics, what cannot be created or destroyed?
Choose one answer.
|
a. Exergy |
||
|
b. Entropy |
||
|
c. Energy |
||
|
d. Enthalpy |
Question 31
In an adiabatic turbine, water vapor at 573°K, 20 bar is expanded to 0.3 bar. What is the amount of work produced per kg vapor?
Choose one answer.
|
a. 256 kJ |
||
|
b. 22 kJ |
||
|
c. 742 kJ |
||
|
d. 1920 kJ |
Question 32
An ideal gas initially at -21°C and 0.334 bar is compressed isothermally to 1.59 bar. Calculate the amount of work done by two moles of gas in this process.
Choose one answer.
|
a. -2.31 kJ |
||
|
b. 4.52 kJ |
||
|
c. -6.53 kJ |
||
|
d. -3.55 kJ |
Question 33
An ideal gas has a molecular weight of 10 kg/k-mole and a specific internal energy of 300 kJ/kg, when the temperature is 373°K. What is the specific enthalpy of this gas?
Choose one answer.
|
a. 610 kJ/kg |
||
|
b. 510 kJ/kg |
||
|
c. 710 kJ/kg |
||
|
d. 210 kJ/kg |
Question 34
Which of the following statements about heat is true?
Choose one answer.
|
a. Heat conduction happens only on solid materials. |
||
|
b. Heat convection is independent of fluid velocity. |
||
|
c. Heat transfer due to radiation increases linearly with temperature. |
||
|
d. None of the above |
Question 35
Which of the following statements about the first law of thermodynamics is false?
Choose one answer.
|
a. The first law of thermodynamics is an expression of the principle of conservation of energy. |
||
|
b. The first law of thermodynamics states that energy can be transformed but cannot be created nor destroyed. |
||
|
c. The internal energy of an isolated system is not constant. |
||
|
d. Changes in internal energy (U) are due to a combination of heat (Q) added to the system and work done by the system (W). |
Question 36
An ideal gas is initially at 60°C and 4 kPa. It then undergoes isobaric expansion, which increases the volume of the gas from 1.6 m3 to 4 m3. What is the amount of work done by the gas in this process?
Choose one answer.
|
a. 3.6 kJ |
||
|
b. 9.6 kJ |
||
|
c. 6.4 kJ |
||
|
d. 5.1 kJ |
Question 37
Which of the following statements is ALWAYS true for an isothermal process of an ideal gas?
Choose one answer.
|
a. The temperature of the system increases. |
||
|
b. The temperature of the system decreases. |
||
|
c. The internal energy of the system stays constant. |
||
|
d. No energy is exchanged between the system and the surrounding. |
Question 38
Which of the following statements is the first law of thermodynamics?
Choose one answer.
|
a. For any spontaneous process, the entropy of the universe increases. |
||
|
b. Entropy cannot be created or destroyed. |
||
|
c. Energy cannot be created or destroyed. |
||
|
d. Energy = internal energy + kinetic energy + potential energy. |
Question 39
Which of the following statements about heat transfer is false?
Choose one answer.
|
a. Heat is always transferred from a hot object to a colder object. |
||
|
b. Heat transfer occurs when the temperature of a system is different from the temperature of its surroundings. |
||
|
c. The three major modes of heat transfer are conduction, convection, and radiation. |
||
|
d. Internal energy of a system can only be changed due to heat transfer. |
Question 40
Which of the following statements about Carnot power cycle is false?
Choose one answer.
|
a. It is not possible to build Carnot power cycle in practice. |
||
|
b. A Carnot power cycle consists of 2 isentropic processes and 2 isothermal processes. |
||
|
c. No engine operating between two heat reservoirs can be more efficient than a Carnot engine operating between those same reservoirs. |
||
|
d. The thermal efficiency of a Rankine cycle can be made to be equal to that of a Carnot power cycle operating between the same cold and hot reservoirs. |
Question 41
What is the thermal efficiency of a Carnot cycle, whose temperatures of the cold and hot reservoirs are 100°C and 500°C, respectively?
Choose one answer.
|
a. 0.13 |
||
|
b. 0.52 |
||
|
c. 0.22 |
||
|
d. 0.76 |
Question 42
Which of the following statements about exergy is true?
Choose one answer.
|
a. Exergy is the minimum useful work possible during a process that brings the system into equilibrium with a heat reservoir. |
||
|
b. Exergy is always destroyed in a process involving a temperature change. |
||
|
c. Exergy is equivalent to entropy. |
||
|
d. Exergy never reaches zero even after the system and surroundings reach equilibrium. |
Question 43
Which of the following statements is the second law of thermodynamics?
Choose one answer.
|
a. For any spontaneous process, the entropy of the universe increases. |
||
|
b. Entropy cannot be created or destroyed. |
||
|
c. Energy cannot be created or destroyed. |
||
|
d. Energy = internal energy + kinetic energy + potential energy. |
Question 44
Which of the following processes are used to form a Carnot cycle?
Choose one answer.
|
a. Two reversible isothermal processes and two adiabatic processes |
||
|
b. Two irreversible isothermal processes and two adiabatic processes |
||
|
c. Two isobaric processes and two adiabatic processes |
||
|
d. Two reversible isentropic processes and two adiabatic processes |
Question 45
Heat is added to a closed system to move the system from state 1 (entropy s1, temperature T1) to state 2 (entropy s2, temperature T2). What is the total amount of heat added to the system in this process if T1 = T2 = T?
Choose one answer.
|
a. T(s1 + s2)/2 |
||
|
b. T (s2 - s1) |
||
|
c. T (s1 - s2) |
||
|
d. T s1/s2 |
Question 46
Which of the following statements about entropy is false?
Choose one answer.
|
a. Entropy expresses the degree of disorder in a system. |
||
|
b. Entropy of an isolated system never decreases. |
||
|
c. Entropy has the dimension of energy divided by temperature and a unit of joules per kelvin (J/K). |
||
|
d. Entropy measures the energy available for useful work in a thermodynamic process. |
Question 47
What is the thermodynamic efficiency of a heat engine that rejects heat at a rate of 20 MW when heat is supplied to it at a rate of 80 MW?
Choose one answer.
|
a. 75% |
||
|
b. 40% |
||
|
c. 65% |
||
|
d. 50% |
Question 48
Which of the following is the thermodynamic quantity that measures the degree of disorder in a system?
Choose one answer.
|
a. Energy |
||
|
b. Entropy |
||
|
c. Work |
||
|
d. Exergy |
Question 49
What is the maximum theoretical efficiency for a heat engine operating between 373°K and 773°K?
Choose one answer.
|
a. 0.123 |
||
|
b. 0.232 |
||
|
c. 0.517 |
||
|
d. 0.721 |
Question 50
Consider a Carnot vapor power cycle using water as the working fluid. Saturated liquid enters the boiler at a pressure of 8 MPa, and saturated vapor enters the turbine. The pressure of the condenser pressure is 8 kPa. What is the thermal efficiency of this power cycle?
Choose one answer.
|
a. 75% |
||
|
b. 35% |
||
|
c. 45% |
||
|
d. 25% |
Question 51
Which of the following processes is NOT a part of a four-stroke Otto power cycle?
Choose one answer.
|
a. Isobaric |
||
|
b. Isothermal |
||
|
c. Adiabatic expansion |
||
|
d. Isochoric |
Question 52
Consider an air-standard Otto cycle, which has a compression ratio of 6. Its temperature and pressure at the beginning of the compression process are 60.33°F and 14.2 lbf/in2, respectively. The heat addition per unit mass of air is 633 kJ. Calculate the thermal efficiency of the cycle.
Choose one answer.
|
a. 76% |
||
|
b. 46% |
||
|
c. 5% |
||
|
d. 29% |
Question 53
What is the thermal efficiency of a Sterling engine which uses an energy source whose temperature is 577°C and an energy sink whose temperature is 57°C?
Choose one answer.
|
a. 0.31 |
||
|
b. 0.71 |
||
|
c. 0.41 |
||
|
d. 0.61 |
Question 54
What is the power cycle that consists of 2 adiabatic processes and 2 isobaric processes?
Choose one answer.
|
a. Carnot |
||
|
b. Brayton |
||
|
c. Diesel |
||
|
d. Otto |
Question 55
What is the power cycle that consists of 2 adiabatic processes and 2 isochoric processes?
Choose one answer.
|
a. Carnot |
||
|
b. Brayton |
||
|
c. Diesel |
||
|
d. Otto |
Question 56
Which of the following power cycles used for internal combustion consists of 2 adiabatic processes, 1 isochoric process, and 1 isobaric process?
Choose one answer.
|
a. Rankine |
||
|
b. Lenoir |
||
|
c. Diesel |
||
|
d. Otto |
Question 57
What is the thermal efficiency of a Rankine cycle if the temperatures of its cold and hot reservoirs are 46°F and 80°F, respectively?
Choose one answer.
|
a. 6.3% |
||
|
b. 17.2% |
||
|
c. 15.3% |
||
|
d. 20.2% |
Question 58
What is the maximum coefficient of performance of a heat pump, whose temperatures of hot and cold reservoirs are 327°C and 27°C, respectively?
Choose one answer.
|
a. 1 |
||
|
b. 0.5 |
||
|
c. 2 |
||
|
d. 4 |
Question 59
An isobaric process of changes temperature of air from T1 = 100°K to T2 = 200°K. If the specific heat of air is 1 kJ/kg-K, what is the change in specific entropy change of the process?
Choose one answer.
|
a. 1.693 kJ/kg-K |
||
|
b. 2.693 kJ/kg-K |
||
|
c. 3.693 kJ/kg-K |
||
|
d. 0.693 kJ/kg-K |
Question 60
A heat engine outputs 300 kW. It receives heat at a rate of 500 kW. The hot and cold temperatures are 2000 K and 300 K, respectively. Calculate the second-law efficiency of the heat engine.
Choose one answer.
|
a. 30% |
||
|
b. 50% |
||
|
c. 71% |
||
|
d. 60% |
Question 61
Calculate the work required to compress 1.5 cubic meter of air initially at room condition (22 °C and 1.02 bar) to 6 bar.
Choose one answer.
|
a. 2.0 MJ |
||
|
b. 1.5 MJ |
||
|
c. 25.3 MJ |
||
|
d. 2.8 MJ |
Question 62
Calculate quality of steam at 8 bar and entropy 6.55 kJ/kg.
Choose one answer.
|
a. 2.4% |
||
|
b. 25% |
||
|
c. 3.8% |
||
|
d. 5.2% |
Question 63
1 kg of dry steam is heated at a constant pressure at 2 bar until its volume is 1.3 m3. Calculate the amount of heat supplied to the process.
Choose one answer.
|
a. 3.5 kJ |
||
|
b. 20 kJ |
||
|
c. 38 kJ |
||
|
d. 336 kJ |
Question 64
A refrigerator is kept in a room with temperature of 95°F. Rate of heat leakage from refrigerator is 1 kW. What is the minimum power needed to keep the temperature of the refrigerator at 10.4 °F?
Choose one answer.
|
a. 1.36 kW |
||
|
b. 2.25 kW |
||
|
c. 3.10 kW |
||
|
d. 0.53 kW |
Question 65
Use van der Waal equation to estimate the volume of 1 kg CO2 at 373 K.
Choose one answer.
|
a. 0.5 m3 |
||
|
b. 2 m3 |
||
|
c. 1 m3 |
||
|
d. 0.1 m3 |
Question 66
Estimate the Rankine efficiency of a steam power cycle. Dry and saturated steam is supplied at 15 bar. The pressure of the condenser is 0.4 bar.
Choose one answer.
|
a. 24% |
||
|
b. 5% |
||
|
c. 72% |
||
|
d. 10% |
Question 67
What is the change in entropy when 1 kmol gas at 10 bar and 300 K expands to a pressure at 1 bar? You can assume that the process is isothermal.
Choose one answer.
|
a. 394 kJ/kmol K |
||
|
b. 19 kJ/kmol K |
||
|
c. 1.5 kJ/kmol K |
||
|
d. 80 kJ/kmol K |
Question 68
Calculate work done when 1 kmol of an ideal gas expanded isothermally at 600 K from 5 bar to 4 bar .
Choose one answer.
|
a. 1113 kJ |
||
|
b. 2312 kJ |
||
|
c. 52 kJ |
||
|
d. 4905 kJ |
Question 69
Consider a constant volume Otto cycle of air (γ = 1.4). The temperature of air at the beginning of the compression is 40°C and the maximum temperature is 2000°C. The compression ratio is 7. Estimate roughly work done per kg air.
Choose one answer.
|
a. 6 kJ |
||
|
b. 600 kJ |
||
|
c. 0.6 kJ |
||
|
d. 6000 kJ |