The general properties and reactivity of each class of organic compounds (Figure 24.1 "Major Classes of Organic Compounds") is largely determined by its functional groups. In this section, we describe the relationships between structure, physical properties, and reactivity for the major classes of organic compounds. We also show you how to apply these relationships to understand some common reactions that chemists use to synthesize organic compounds.
The boiling points of alkanes increase smoothly with increasing molecular mass. They are similar to those of the corresponding alkenes and alkynes because of similarities in molecular mass between analogous structures (Table 24.1 "Boiling Points (in °C) of Alkanes, Alkenes, and Alkynes of Comparable Molecular Mass"). In contrast, the melting points of alkanes, alkenes, and alkynes with similar molecular masses show a much wider variation because the melting point strongly depends on how the molecules stack in the solid state. It is therefore sensitive to relatively small differences in structure, such as the location of a double bond and whether the molecule is cis or trans.
Table 24.1 Boiling Points (in °C) of Alkanes, Alkenes, and Alkynes of Comparable Molecular Mass
| Length of Carbon Chain | |||
|---|---|---|---|
| Class | Two C Atoms | Three C Atoms | Four C Atoms |
| alkane | −88.6 | −42.1 | −0.5 |
| alkene | −103.8 | −47.7 | −6.3 |
| alkyne | −84.7 | −23.2 | 8.1 |
Because alkanes contain only C–C and C–H bonds, which are strong and not very polar (the electronegativities of C and H are similar; Figure 7.15 "Pauling Electronegativity Values of the "), they are not easily attacked by nucleophiles or electrophiles. Consequently, their reactivity is limited, and often their reactions occur only under extreme conditions. For example, catalytic cracking can be used to convert straight-chain alkanes to highly branched alkanes, which are better fuels for internal combustion engines. Catalytic cracking is one example of a pyrolysis reactionA high-temperature decomposition reaction that can be used to form fibers of synthetic polymers. (from the Greek pyros, meaning “fire,” and lysis, meaning “loosening”), in which alkanes are heated to a sufficiently high temperature to induce cleavage of the weakest bonds: the C–C single bonds. The result is a mixture of radicals derived from essentially random cleavage of the various C–C bonds in the chain. Pyrolysis of n-pentane, for example, is nonspecific and can produce these four radicals:
Equation 24.7
Recombination of these radicals (a termination step) can produce ethane, propane, butane, n-pentane, n-hexane, n-heptane, and n-octane. Radicals that are formed in the middle of a chain by cleaving a C–H bond tend to produce branched hydrocarbons. In catalytic cracking, lighter alkanes are removed from the mixture by distillation.
Radicals are also produced during the combustion of alkanes, with CO2 and H2O as the final products. As discussed in Section 24.3 "Reactivity of Organic Molecules", radicals are stabilized by the presence of multiple carbon substituents that can donate electron density to the electron-deficient carbon. The chemical explanation of octane ratings, as described in Chapter 2 "Molecules, Ions, and Chemical Formulas", Section 2.6 "Industrially Important Chemicals", rests partly on the stability of radicals produced from the different hydrocarbon fuels. Recall that n-heptane, which does not burn smoothly, has an octane rating of 0, and 2,2,4-trimethylpentane (“isooctane”), which burns quite smoothly, has a rating of 100 (Figure 2.25 "The Octane Ratings of Some Hydrocarbons and Common Additives"). Isooctane has a branched structure and is capable of forming tertiary radicals that are comparatively stable.
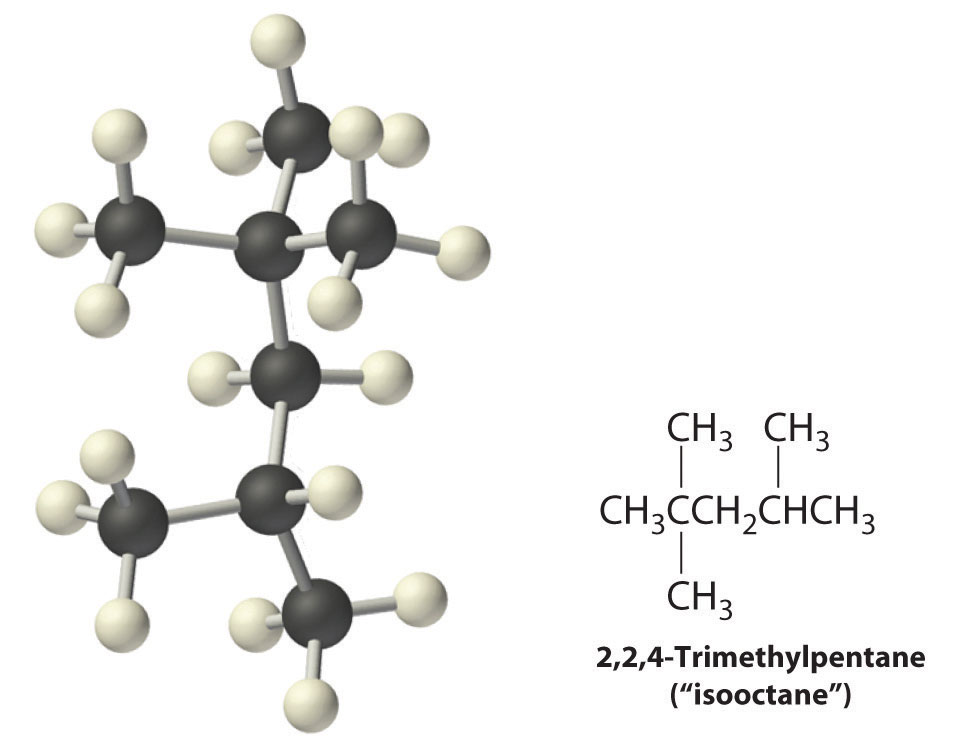
In contrast, the radicals formed during the combustion of n-heptane, whether primary or secondary, are less stable and hence more reactive, which partly explains why burning n-heptane causes premature ignition and engine knocking.
In Section 24.2 "Isomers of Organic Compounds", we explained that rotation about the carbon–carbon multiple bonds of alkenes and alkynes cannot occur without breaking a π bond, which therefore constitutes a large energy barrier to rotation (Figure 24.15 "Carbon–Carbon Bonding in Alkenes and Interconversion of "). Consequently, the cis and trans isomers of alkenes generally behave as distinct compounds with different chemical and physical properties. A four-carbon alkene has four possible isomeric forms: three structural isomers, which differ in their connectivity, plus a pair of geometric isomers from one structural isomer (2-butene). These two geometric isomers are cis-2-butene and trans-2-butene. The four isomers have significantly different physical properties.
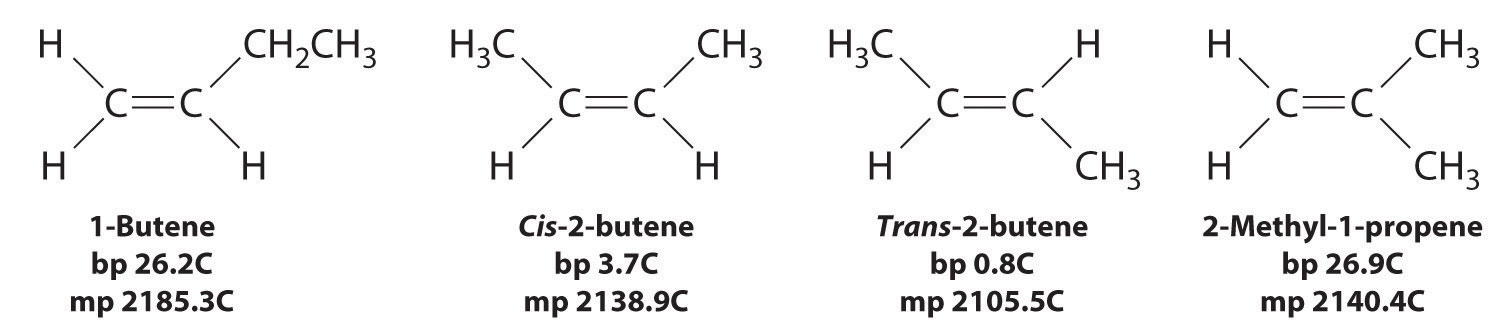
Figure 24.15 Carbon–Carbon Bonding in Alkenes and Interconversion of Cis and Trans Isomers
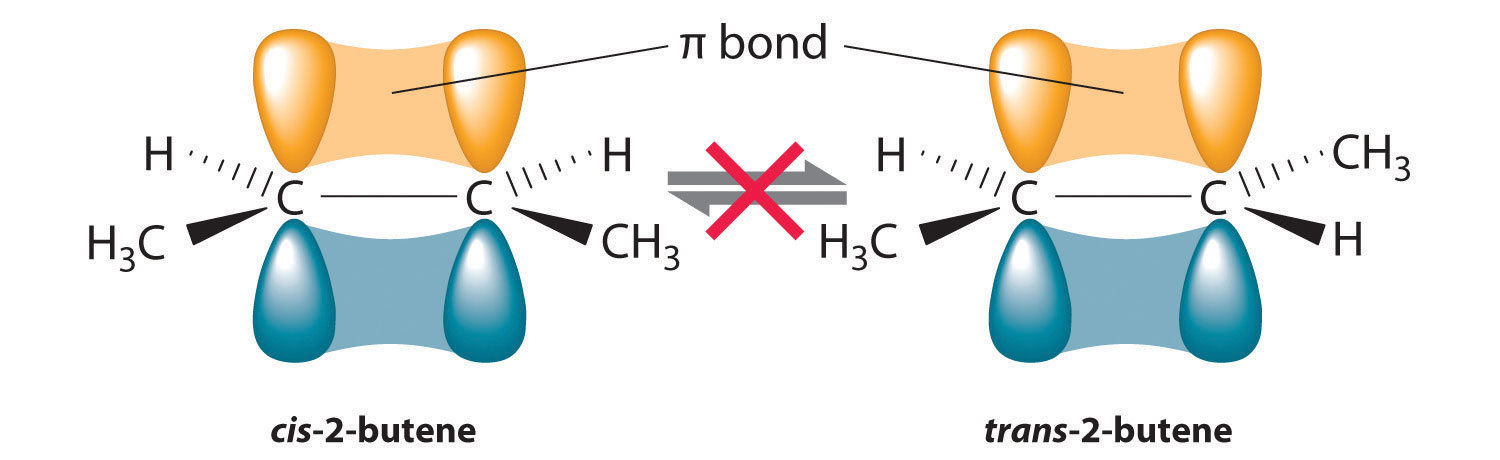
In butane, there is only a small energy barrier to rotation about the C2–C3 σ bond. In the formation of cis- or trans-2-butene from butane, the p orbitals on C2 and C3 overlap to form a π bond. To convert cis-2-butene to trans-2-butene or vice versa through rotation about the double bond, the π bond must be broken. Because this interconversion is energetically unfavorable, cis and trans isomers are distinct compounds that generally have different physical and chemical properties.
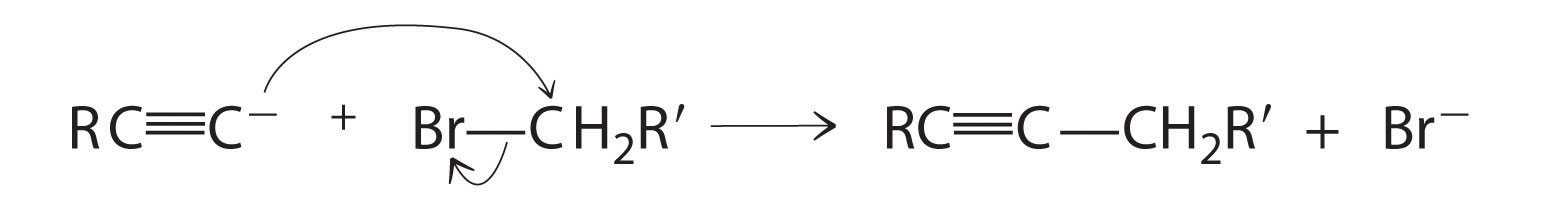
Alkynes in which the triple bond is located at one end of a carbon chain are called terminal alkynes and contain a hydrogen atom attached directly to a triply bonded carbon: R–C≡C–H. Terminal alkynes are unusual in that the hydrogen atom can be removed relatively easily as H+, forming an acetylide ion (R–C≡C−). Acetylide ions are potent nucleophiles that are especially useful reactants for making longer carbon chains by a nucleophilic substitution reaction. As in earlier examples of such reactions, the nucleophile attacks the partially positively charged atom in a polar bond, which in the following reaction is the carbon of the Br–C bond:
Alkenes and alkynes are most often prepared by elimination reactions (Figure 24.13). A typical example is the preparation of 2-methyl-1-propene, whose derivative, 3-chloro-2-methyl-1-propene, is used as a fumigant and insecticide. The parent compound can be prepared from either 2-hydroxy-2-methylpropane or 2-bromo-2-methylpropane:
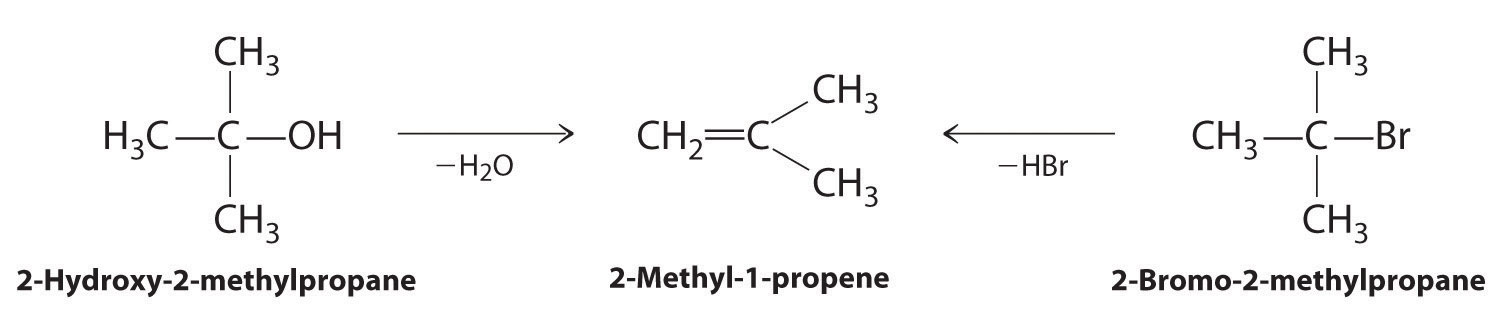
The reaction on the left proceeds by eliminating the elements of water (H+ plus OH−), so it is a dehydration reactionA reaction that proceeds by eliminating the elements of water . If an alkane contains two properly located functional groups, such as –OH or –X, both of them may be removed as H2O or HX with the formation of a carbon–carbon triple bond:
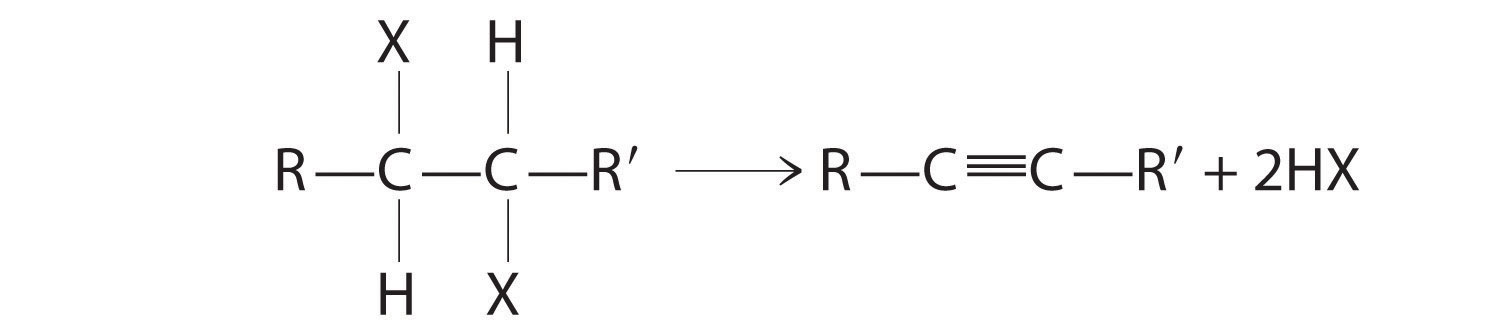
Alkenes and alkynes are most often prepared by elimination reactions.
Most arenes that contain a single six-membered ring are volatile liquids, such as benzene and the xylenes, although some arenes with substituents on the ring are solids at room temperature. In the gas phase, the dipole moment of benzene is zero, but the presence of electronegative or electropositive substituents can result in a net dipole moment that increases intermolecular attractive forces and raises the melting and boiling points. For example, 1,4-dichlorobenzene, a compound used as an alternative to naphthalene in the production of mothballs, has a melting point of 52.7°C, which is considerably greater than the melting point of benzene (5.5°C). (For more information on 1,4-dichlorobenzene, see Chapter 11 "Liquids", Section 11.5 "Changes of State".)
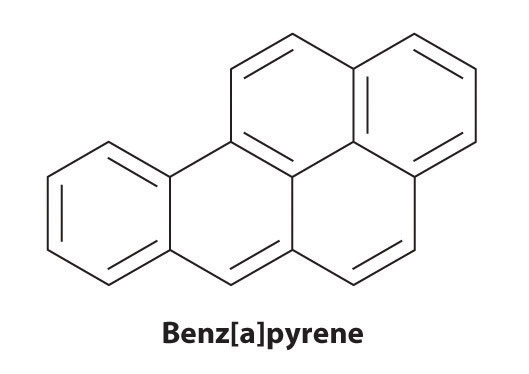
Certain aromatic hydrocarbons, such as benzene and benz[a]pyrene, are potent liver toxins and carcinogens. In 1775, a British physician, Percival Pott, described the high incidence of cancer of the scrotum among small boys used as chimney sweeps and attributed it to their exposure to soot. His conclusions were correct: benz[a]pyrene, a component of chimney soot, charcoal-grilled meats, and cigarette smoke, was the first chemical carcinogen to be identified.
Although arenes are usually drawn with three C=C bonds, benzene is about 150 kJ/mol more stable than would be expected if it contained three double bonds. This increased stability is due to the delocalization of the π electron density over all the atoms of the ring. (For more information on delocalization, see Chapter 9 "Molecular Geometry and Covalent Bonding Models", Section 9.3 "Delocalized Bonding and Molecular Orbitals".) Compared with alkenes, arenes are poor nucleophiles. Consequently, they do not undergo addition reactions like alkenes; instead, they undergo a variety of electrophilic aromatic substitution reactionsA reaction in which a −H of an arene is replaced (substituted) by an electrophilic group in a two-step process. that involve the replacement of –H on the arene by a group –E, such as –NO2, –SO3H, a halogen, or an alkyl group, in a two-step process. The first step involves addition of the electrophile (E) to the π system of benzene, forming a carbocation. In the second step, a proton is lost from the adjacent carbon on the ring:
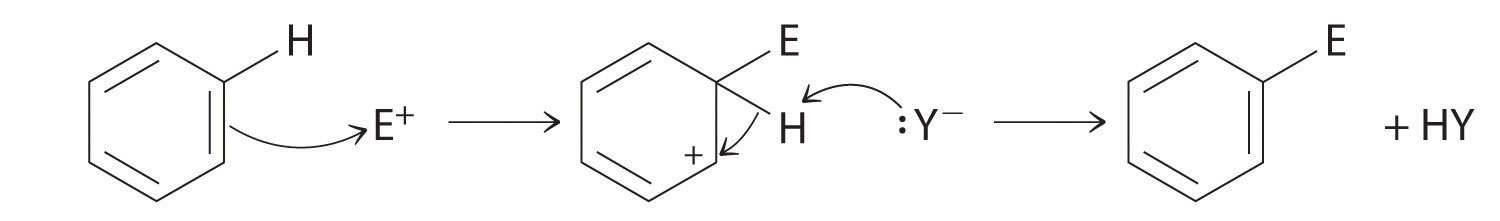
The carbocation formed in the first step is stabilized by resonance.
Arenes undergo substitution reactions rather than elimination because of increased stability arising from delocalization of their π electron density.
Many substituted arenes have potent biological activity. Some examples include common drugs and antibiotics such as aspirin and ibuprofen, illicit drugs such as amphetamines and peyote, the amino acid phenylalanine, and hormones such as adrenaline (Figure 24.16 "Biologically Active Substituted Arenes").
Figure 24.16 Biologically Active Substituted Arenes
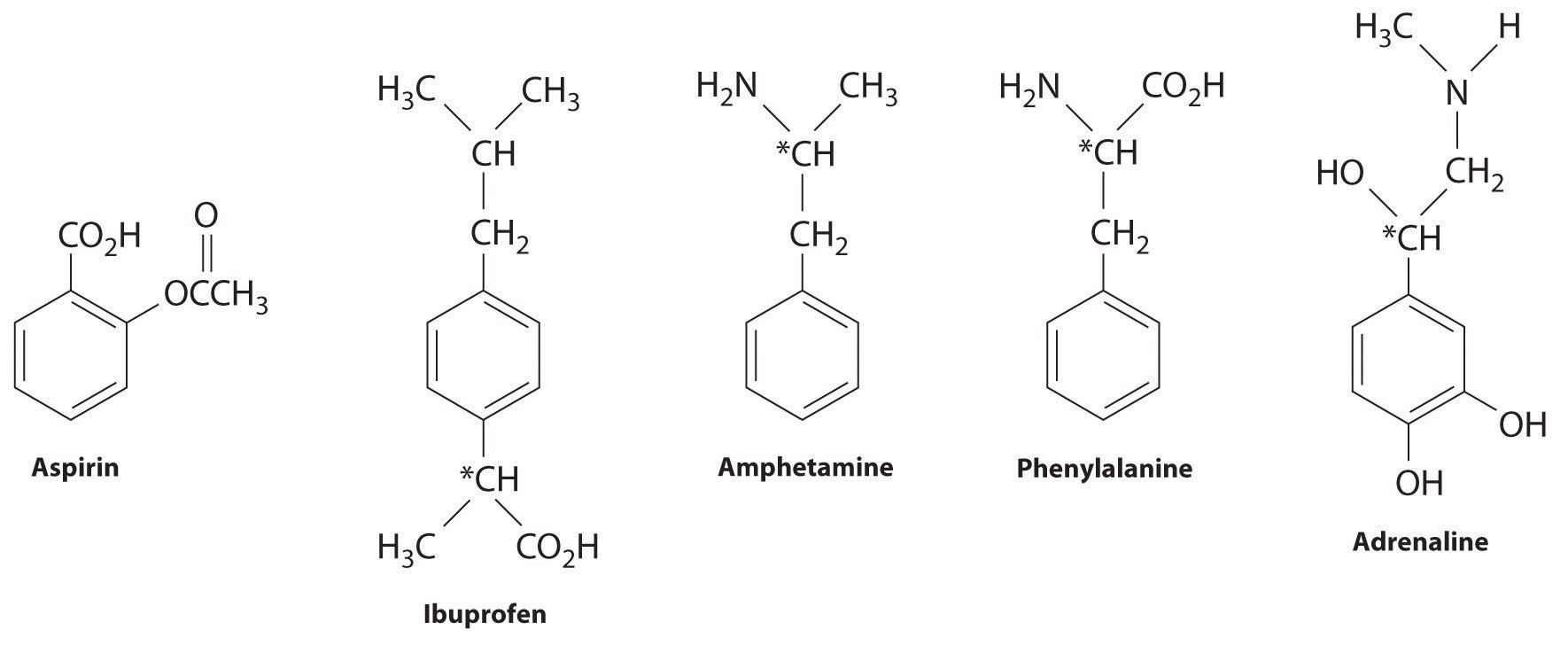
Aspirin (antifever activity), ibuprofen (antifever and anti-inflammatory activity), and amphetamine (stimulant) have pharmacological effects. Phenylalanine is an amino acid. Adrenaline is a hormone that elicits the “fight or flight” response to stress. Chiral centers are indicated with an asterisk.
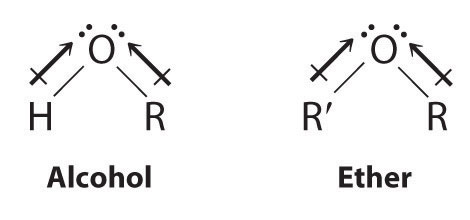
Both alcohols and ethers can be thought of as derivatives of water in which at least one hydrogen atom has been replaced by an organic group, as shown here. Because of the electronegative oxygen atom, the individual O–H bond dipoles in alcohols cannot cancel one another, resulting in a substantial dipole moment that allows alcohols to form hydrogen bonds. Alcohols therefore have significantly higher boiling points than alkanes or alkenes of comparable molecular mass, whereas ethers, without a polar O–H bond, have intermediate boiling points due to the presence of a small dipole moment (Table 24.2 "Boiling Points of Alkanes, Ethers, and Alcohols of Comparable Molecular Mass"). The larger the alkyl group in the molecule, however, the more “alkane-like” the alcohol is in its properties. Because of their polar nature, alcohols and ethers tend to be good solvents for a wide range of organic compounds.
Table 24.2 Boiling Points of Alkanes, Ethers, and Alcohols of Comparable Molecular Mass
| Name | Formula | Molecular Mass (amu) | Boiling Point (°C) | |
|---|---|---|---|---|
| alkane | propane | C3H8 | 44 | −42.1 |
| n-pentane | C5H12 | 72 | 36.1 | |
| n-heptane | C7H16 | 100 | 98.4 | |
| ether | dimethylether | (CH3)2O | 46 | −24.8 |
| diethylether | (CH3CH2)2O | 74 | 34.5 | |
| di-n-propylether | (CH3CH2CH2)2O | 102 | 90.1 | |
| alcohol | ethanol | CH3CH2OH | 46 | 78.3 |
| n-butanol | CH3(CH2)3OH | 74 | 117.7 | |
| n-hexanol | CH3(CH2)5OH | 102 | 157.6 |
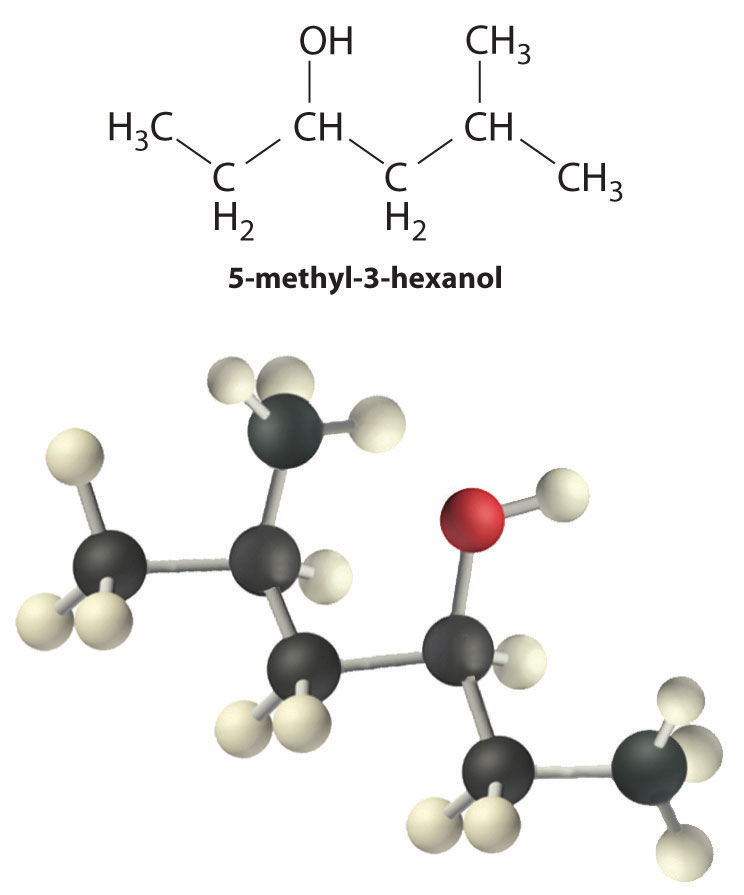
Alcohols are usually prepared by adding water across a carbon–carbon double bond or by a nucleophilic substitution reaction of an alkyl halide using hydroxide, a potent nucleophile (Figure 24.12). As you will see in Section 24.6 "The Molecules of Life", alcohols can also be prepared by reducing compounds that contain the carbonyl functional group (C=O; part (a) in Figure 24.14 "The Oxidation State of Carbon in Oxygen- and Nitrogen-Containing Functional Groups"). Alcohols are classified as primary, secondary, or tertiary, depending on whether the –OH group is bonded to a primary, secondary, or tertiary carbon. For example, the compound 5-methyl-3-hexanol is a secondary alcohol.
Ethers, especially those with two different alkyl groups (ROR′), can be prepared by a substitution reaction in which a nucleophilic alkoxide ion (RO−) attacks the partially positively charged carbon atom of the polar C–X bond of an alkyl halide (R′X):
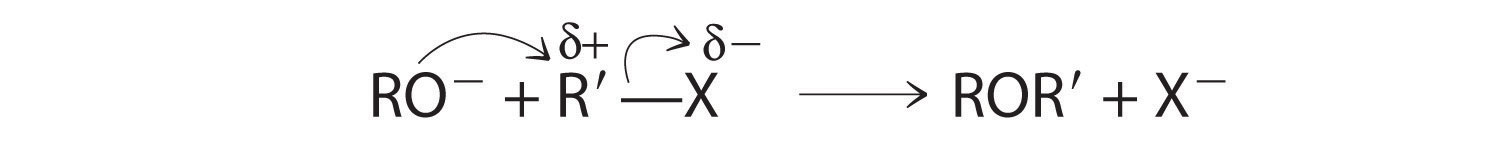
Although both alcohols and phenols have an –OH functional group, phenols are 106–108 more acidic than alcohols. This is largely because simple alcohols have the –OH unit attached to an sp3 hybridized carbon, whereas phenols have an sp2 hybridized carbon atom bonded to the oxygen atom. The negative charge of the phenoxide ion can therefore interact with the π electrons in the ring, thereby delocalizing and stabilizing the negative charge through resonance. (For more information on resonance, see Chapter 8 "Ionic versus Covalent Bonding", Section 8.5 "Lewis Structures and Covalent Bonding".) In contrast, the negative charge on an alkoxide ion cannot be stabilized by these types of interactions.
Alcohols undergo two major types of reactions: those involving cleavage of the O–H bond and those involving cleavage of the C–O bond. Cleavage of an O–H bond is a reaction characteristic of an acid, but alcohols are even weaker acids than water. The acidic strength of phenols, however, is about a million times greater than that of ethanol, making the pKa of phenol comparable to that of the NH4+ ion (9.89 versus 9.25, respectively):
Equation 24.8
Alcohols undergo two major types of reactions: cleavage of the O–H bond and cleavage of the C–O bond.
Cleavage of the C–O bond in alcohols occurs under acidic conditions. The –OH is first protonated, and nucleophilic substitution follows:
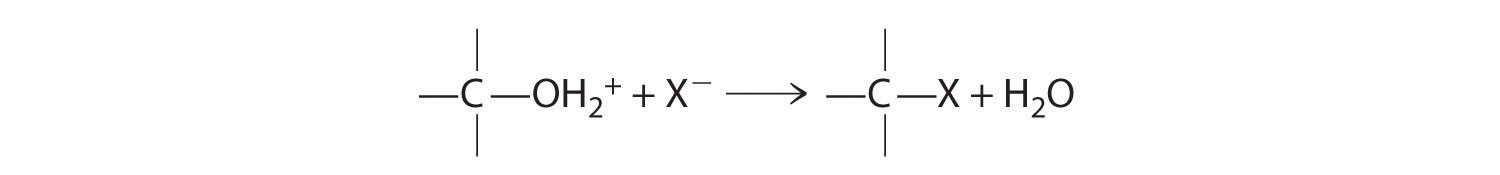
In the absence of a nucleophile, however, elimination can occur, producing an alkene (Figure 24.13).
Ethers lack the –OH unit that is central to the reactivity of alcohols, so they are comparatively unreactive. Their low reactivity makes them highly suitable as solvents for carrying out organic reactions.
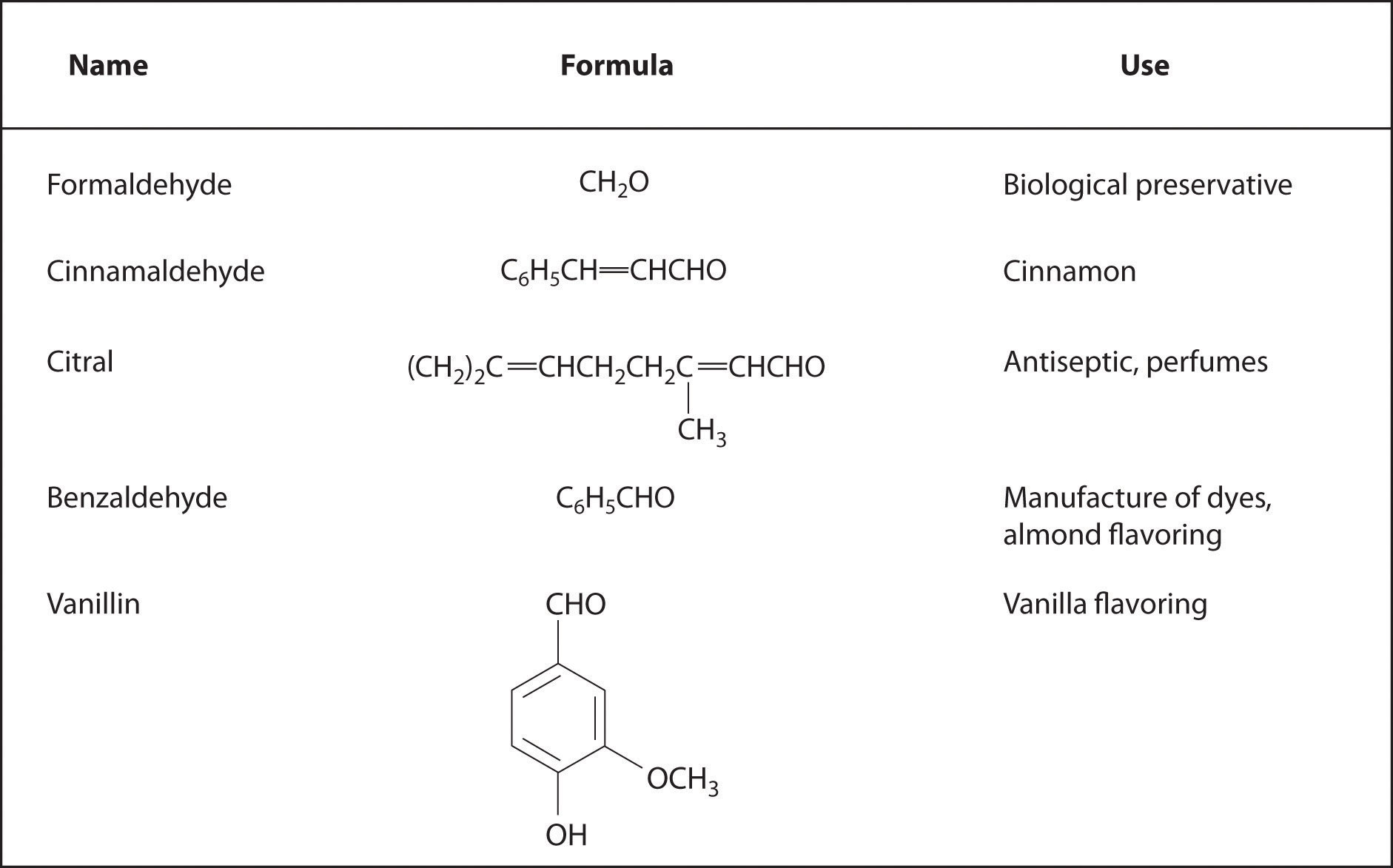
Aromatic aldehydes, which have intense and characteristic flavors and aromas, are the major components of such well-known flavorings as vanilla and cinnamon (Figure 24.17 "Some Familiar Aldehydes and Their Uses"). Many ketones, such as camphor and jasmine, also have intense aromas. Ketones are found in many of the hormones responsible for sex differentiation in humans, such as progesterone and testosterone. (For more information on aldehydes and ketones, see Chapter 4 "Reactions in Aqueous Solution", Section 4.1 "Aqueous Solutions".)
Figure 24.17 Some Familiar Aldehydes and Their Uses
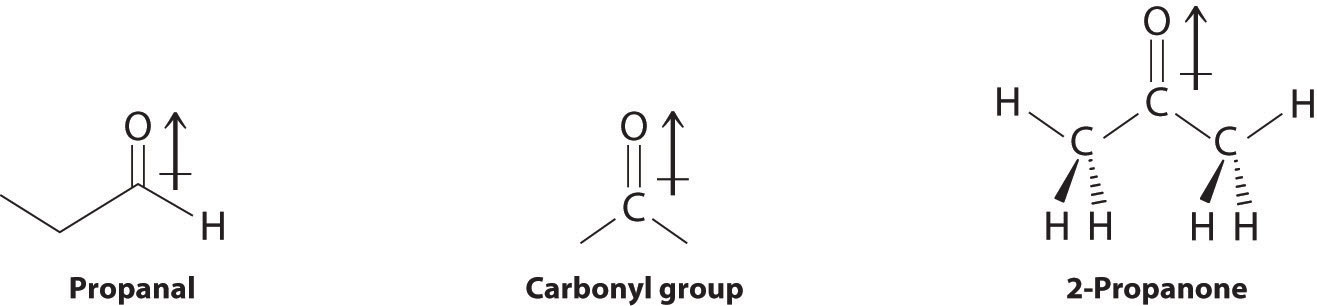
In compounds containing a carbonyl group, nucleophilic attack can occur at the carbon atom of the carbonyl, whereas electrophilic attack occurs at oxygen.
Aldehydes and ketones contain the carbonyl functional group, which has an appreciable dipole moment because of the polar C=O bond. The presence of the carbonyl group results in strong intermolecular interactions that cause aldehydes and ketones to have higher boiling points than alkanes or alkenes of comparable molecular mass (Table 24.3 "Boiling Points of Alkanes, Aldehydes, and Ketones of Comparable Molecular Mass"). As the mass of the molecule increases, the carbonyl group becomes less important to the overall properties of the compound, and the boiling points approach those of the corresponding alkanes.
Table 24.3 Boiling Points of Alkanes, Aldehydes, and Ketones of Comparable Molecular Mass
| Name | Formula | Molecular Mass (amu) | Boiling Point (°C) | |
|---|---|---|---|---|
| alkane | n-butane | C4H10 | 58 | −0.5 |
| n-pentane | C5H12 | 72 | 36.1 | |
| aldehyde | propionaldehyde (propanal) | C3H6O | 58 | 48.0 |
| butyraldehyde (butanal) | C4H8O | 72 | 74.8 | |
| ketone | acetone (2-propanone) | C3H6O | 58 | 56.1 |
| methyl ethyl ketone (2-butanone) | C4H8O | 72 | 79.6 |
Aldehydes and ketones are typically prepared by oxidizing alcohols (part (a) in Figure 24.14 "The Oxidation State of Carbon in Oxygen- and Nitrogen-Containing Functional Groups"). In their reactions, the partially positively charged carbon atom of the carbonyl group is an electrophile that is subject to nucleophilic attack. Conversely, the lone pairs of electrons on the oxygen atom of the carbonyl group allow electrophilic attack to occur. Aldehydes and ketones can therefore undergo both nucleophilic attack (at the carbon atom) and electrophilic attack (at the oxygen atom).
Nucleophilic attack occurs at the partially positively charged carbon of a carbonyl functional group. Electrophilic attack occurs at the lone pairs of electrons on the oxygen atom.
Aldehydes and ketones react with many organometallic compounds that contain stabilized carbanions. One of the most important classes of such compounds are the Grignard reagentsAn organometallic compound that has stabilized carbanions, whose general formula is RMgX, where X is Cl, Br, or I., organomagnesium compounds with the formula RMgX (X is Cl, Br, or I) that are so strongly polarized that they can be viewed as containing R− and MgX+. These reagents are named for the French chemist Victor Grignard (1871–1935), who won a Nobel Prize in Chemistry in 1912 for their development. In a Grignard reaction, the carbonyl functional group is converted to an alcohol, and the carbon chain of the carbonyl compound is lengthened by the addition of the R group from the Grignard reagent. One example is reacting cyclohexylmagnesium chloride, a Grignard reagent, with formaldehyde:
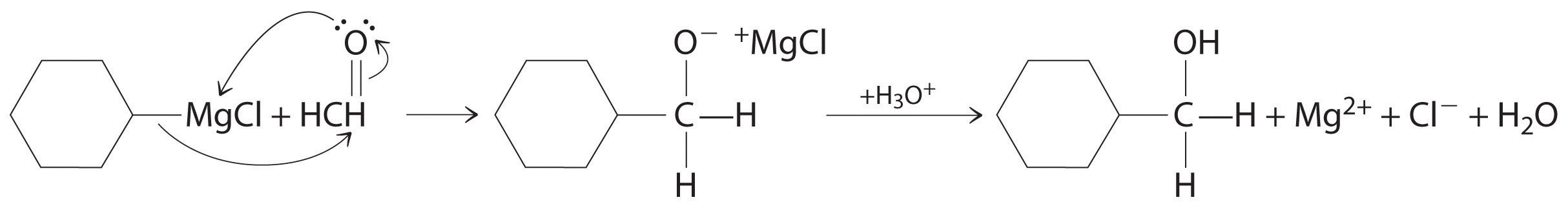
The nucleophilic carbanion of the cyclohexyl ring attacks the electrophilic carbon atom of the carbonyl group. Acidifying the solution results in protonation of the intermediate to give the alcohol. Aldehydes can also be prepared by reducing a carboxylic acid group (–CO2H) (part (a) in Figure 24.14 "The Oxidation State of Carbon in Oxygen- and Nitrogen-Containing Functional Groups"), and ketones can be prepared by reacting a carboxylic acid derivative with a Grignard reagent. The former reaction requires a powerful reducing agent, such as a metal hydride.
Explain how each reaction proceeds to form the indicated product.
Given: chemical reaction
Asked for: how products are formed
Strategy:
A Identify the functional group and classify the reaction.
B Use the mechanisms described to propose the initial steps in the reaction.
Solution:
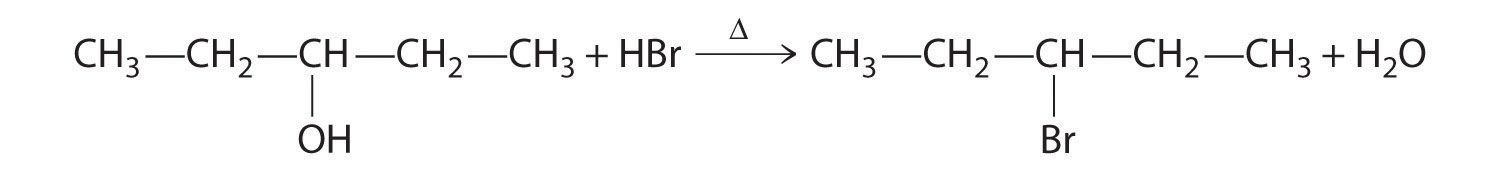
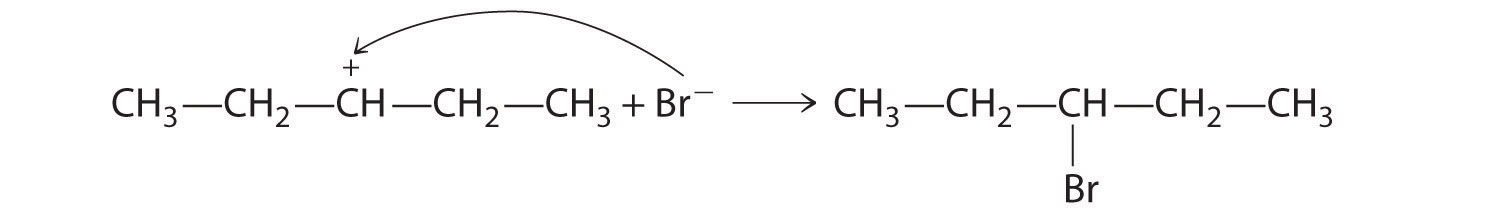
A One reactant is an alcohol that undergoes a substitution reaction.
B In the product, a bromide group is substituted for a hydroxyl group. The first step in this reaction must therefore be protonation of the –OH group of the alcohol by H+ of HBr, followed by the elimination of water to give the carbocation:
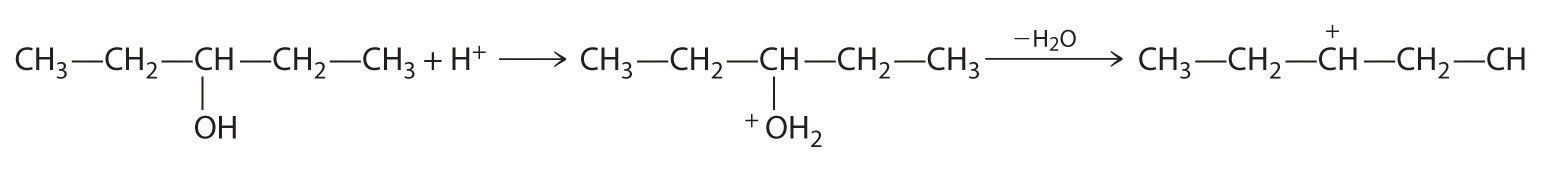
The bromide ion is a good nucleophile that can react with the carbocation to give an alkyl bromide:
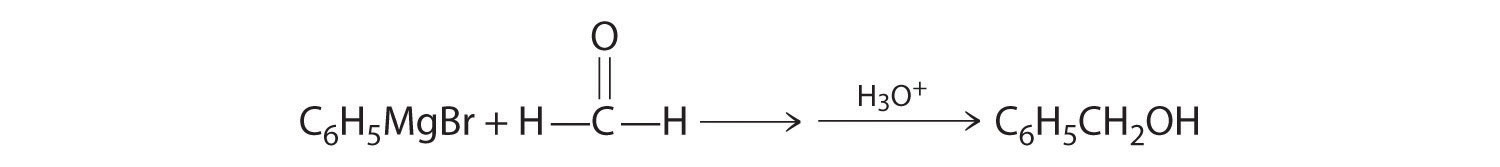
A One reactant is a Grignard reagent, and the other contains a carbonyl functional group. Carbonyl compounds act as electrophiles, undergoing nucleophilic attack at the carbonyl carbon.
B The nucleophile is the phenyl carbanion of the Grignard reagent:
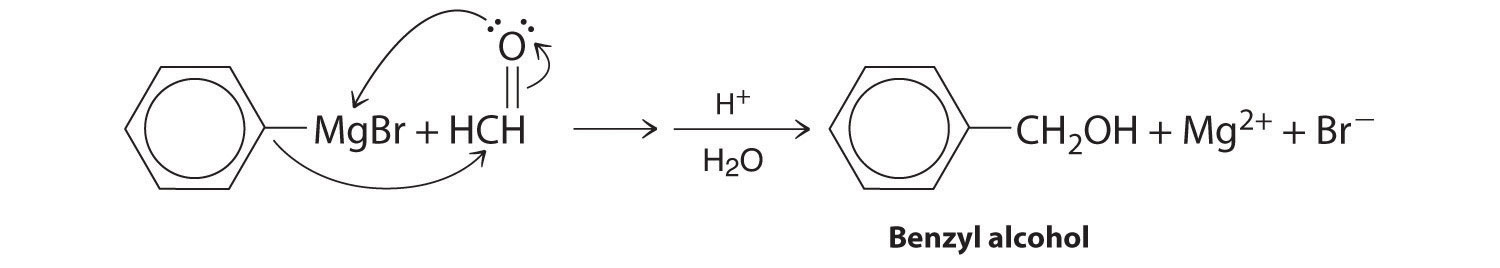
The product is benzyl alcohol.
Exercise
Predict the product of each reaction.
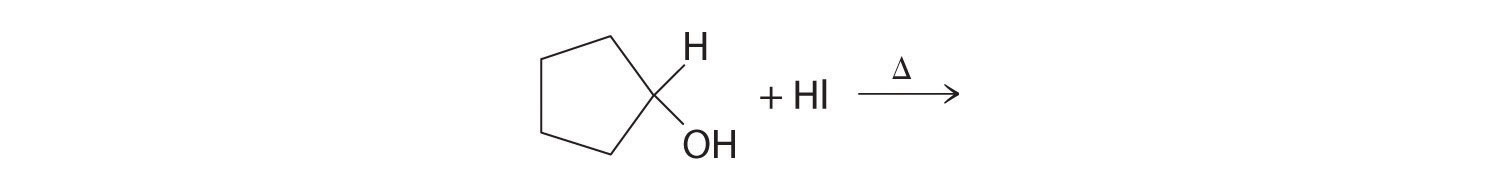
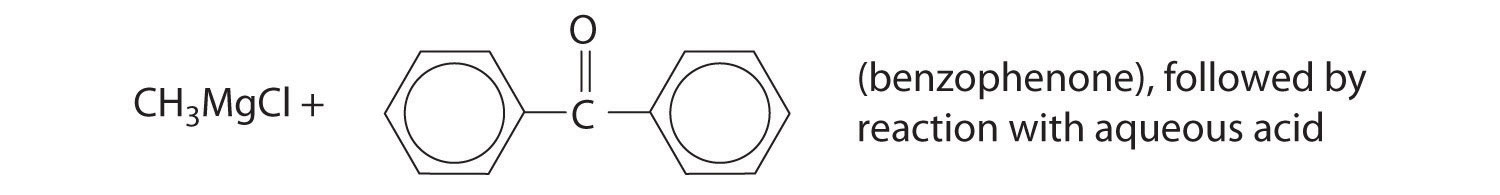
Answer:
The pungent odors of many carboxylic acids are responsible for the smells we associate with sources as diverse as Swiss cheese, rancid butter, manure, goats, and sour milk. The boiling points of carboxylic acids tend to be somewhat higher than would be expected from their molecular masses because of strong hydrogen-bonding interactions between molecules. In fact, most simple carboxylic acids form dimers in the liquid and even in the vapor phase. (For more information on the vapor phase, see Chapter 11 "Liquids", Section 11.2 "Intermolecular Forces".) The four lightest carboxylic acids are completely miscible with water, but as the alkyl chain lengthens, they become more “alkane-like,” so their solubility in water decreases.
Compounds that contain the carboxyl functional group are acidic because carboxylic acids can easily lose a proton: the negative charge in the carboxylate ion (RCO2−) is stabilized by delocalization of the π electrons:
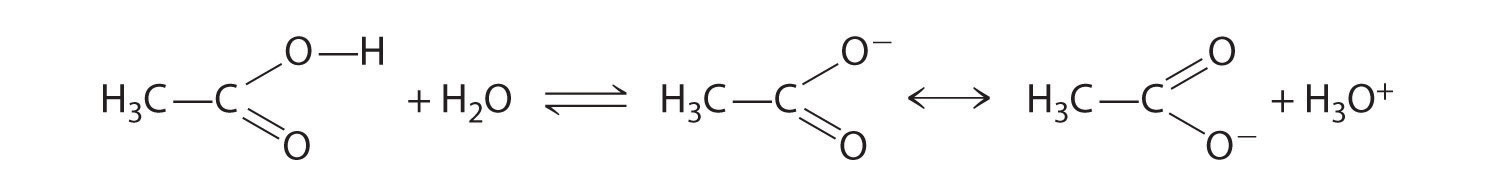
As a result, carboxylic acids are about 1010 times more acidic than the corresponding simple alcohols whose anions (RO−) are not stabilized through resonance.
Carboxylic acids are typically prepared by oxidizing the corresponding alcohols and aldehydes (part (a) in Figure 24.14 "The Oxidation State of Carbon in Oxygen- and Nitrogen-Containing Functional Groups"). They can also be prepared by reacting a Grignard reagent with CO2, followed by acidification:
Equation 24.9
The initial step in the reaction is nucleophilic attack by the R− group of the Grignard reagent on the electrophilic carbon of CO2:
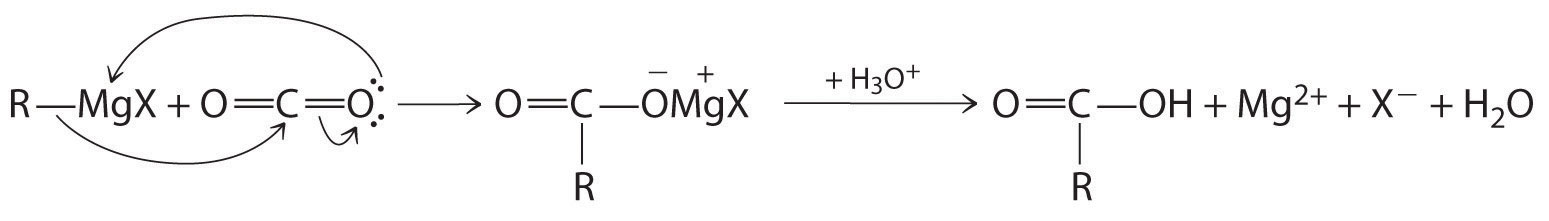
Delocalization of π bonding over three atoms (O–C–O) makes carboxylic acids and their derivatives less susceptible to nucleophilic attack than aldehydes and ketones with their single π bond. The reactions of carboxylic acids are dominated by two factors: their polar –CO2H group and their acidity. Reaction with strong bases, for example, produce carboxylate salts, such as sodium stearate:
Equation 24.10
RCO2H + NaOH → RCO2−Na+ + H2Owhere R is CH3(CH2)16. As you learned in Chapter 13 "Solutions", Section 13.6 "Aggregate Particles in Aqueous Solution", long-chain carboxylate salts are used as soaps.
Delocalization of π bonding over three atoms makes carboxylic acids and their derivatives less susceptible to nucleophilic attack as compared with aldehydes and ketones.
Replacing the –OH of a carboxylic acid with groups that have different tendencies to participate in resonance with the C=O functional group produces derivatives with rather different properties. Resonance structures have significant effects on the reactivity of carboxylic acid derivatives, but their influence varies substantially, being least important for halides and most important for the nitrogen of amides. In this section, we take a brief look at the chemistry of two of the most familiar and important carboxylic acid derivatives: esters and amides.
Esters have the general formula RCO2R′, where R and R′ can be virtually any alkyl or aryl group. Esters are often prepared by reacting an alcohol (R′OH) with a carboxylic acid (RCO2H) in the presence of a catalytic amount of strong acid. (For more information on esters and catalysts, see Chapter 3 "Chemical Reactions", Section 3.5 "Classifying Chemical Reactions".) The purpose of the acid (an electrophile) is to protonate the doubly bonded oxygen atom of the carboxylic acid (a nucleophile) to give a species that is more electrophilic than the parent carboxylic acid.
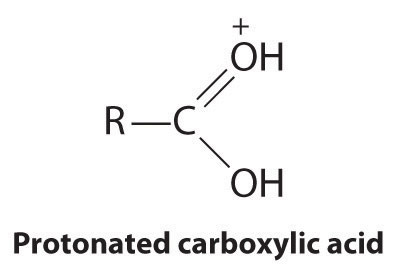
The nucleophilic oxygen atom of the alcohol attacks the electrophilic carbon atom of the protonated carboxylic acid to form a new C–O bond. The overall reaction can be written as follows:
Figure 24.18
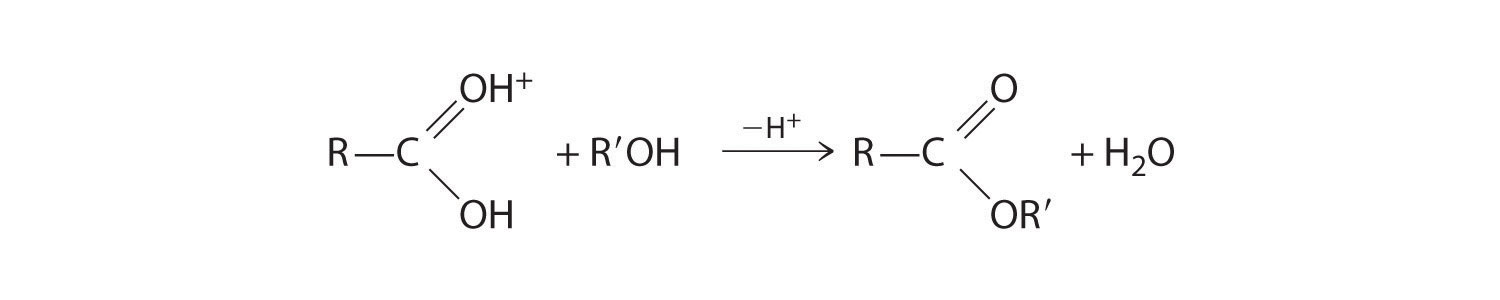
Because water is eliminated, this is a dehydration reaction. If an aqueous solution of an ester and strong acid or base is heated, the reverse reaction will occur, producing the parent alcohol R′OH and either the carboxylic acid RCO2H (under strongly acidic conditions) or the carboxylate anion RCO2− (under basic conditions).
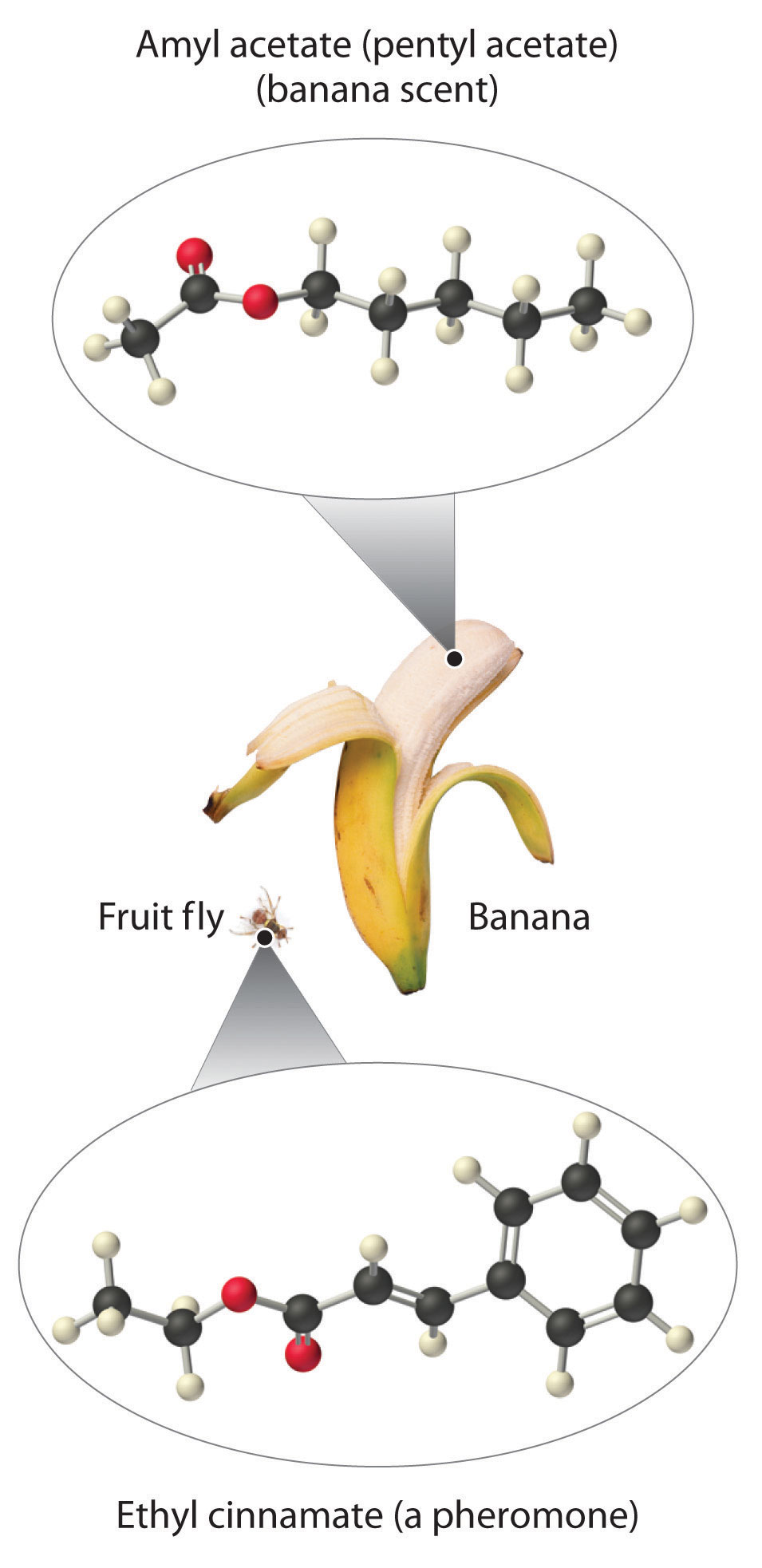
As stated earlier, esters are familiar to most of us as fragrances, such as banana and pineapple. Other esters with intense aromas function as sex attractants, or pheromones, such as the pheromone from the oriental fruit fly. Research on using synthetic insect pheromones as a safer alternative to insecticides for controlling insect populations, such as cockroaches, is a rapidly growing field in organic chemistry.
In the general structure of an amide,
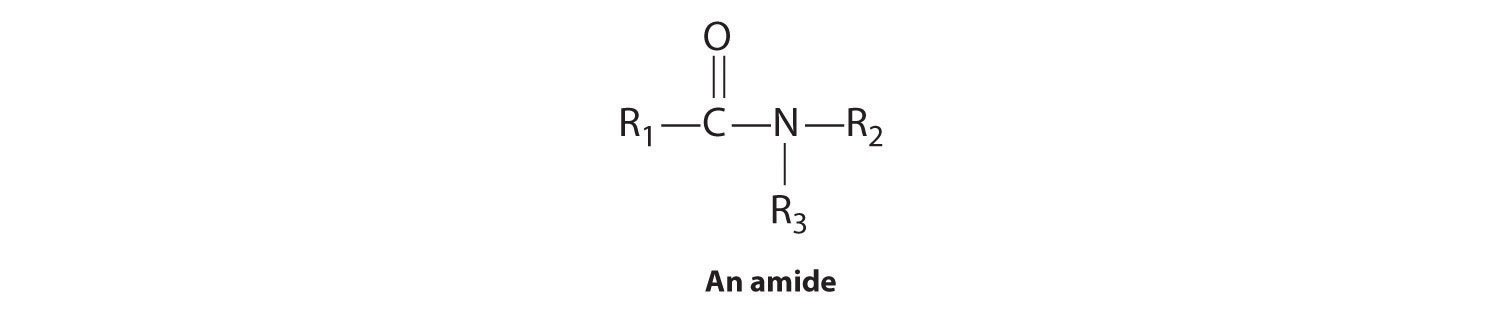
the two substituents on the amide nitrogen can be hydrogen atoms, alkyl groups, aryl groups, or any combination of those species. Although amides appear to be derived from an acid and an amine, in practice they usually cannot be prepared by this synthetic route. In principle, nucleophilic attack by the lone electron pair of the amine on the carbon of the carboxylic acid could occur, but because carboxylic acids are weak acids and amines are weak bases, an acid–base reaction generally occurs instead:
Equation 24.11
RCO2H + R′NH2 → RCO2− + R′NH3+Amides are therefore usually prepared by the nucleophilic reaction of amines with more electrophilic carboxylic acid derivatives, such as esters.
The lone pair of electrons on the nitrogen atom of an amide can participate in π bonding with the carbonyl group, thus reducing the reactivity of the amide (Figure 24.19 "The Electronic Structure of an Amide") and inhibiting free rotation about the C–N bond. Amides are therefore the least reactive of the carboxylic acid derivatives. The stability of the amide bond is crucially important in biology because amide bonds form the backbones of peptides and proteins. (For more information on peptides and proteins, see Chapter 12 "Solids", Section 12.8 "Polymeric Solids".) The amide bond is also found in many other biologically active and commercially important molecules, including penicillin; urea, which is used as fertilizer; saccharin, a sugar substitute; and valium, a potent tranquilizer. (For more information on the structure of penicillin, see Chapter 3 "Chemical Reactions", Section 3.2 "Determining Empirical and Molecular Formulas".)
Amides are the least reactive of the carboxylic acid derivatives because amides participate in π bonding with the carbonyl group.
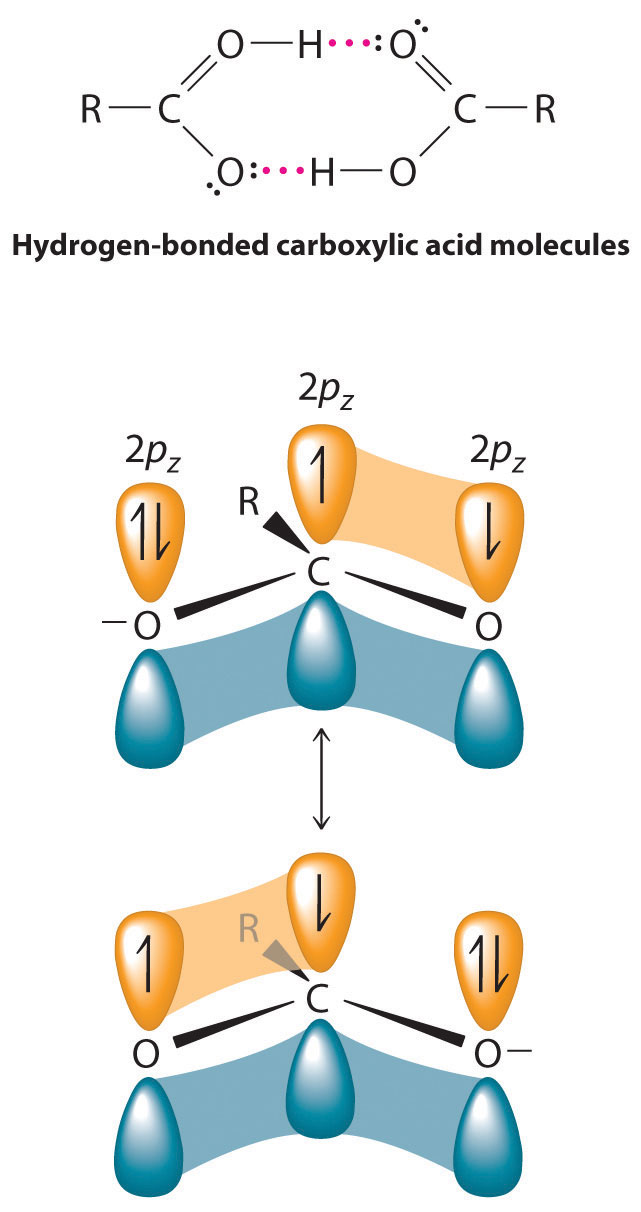
Figure 24.19 The Electronic Structure of an Amide
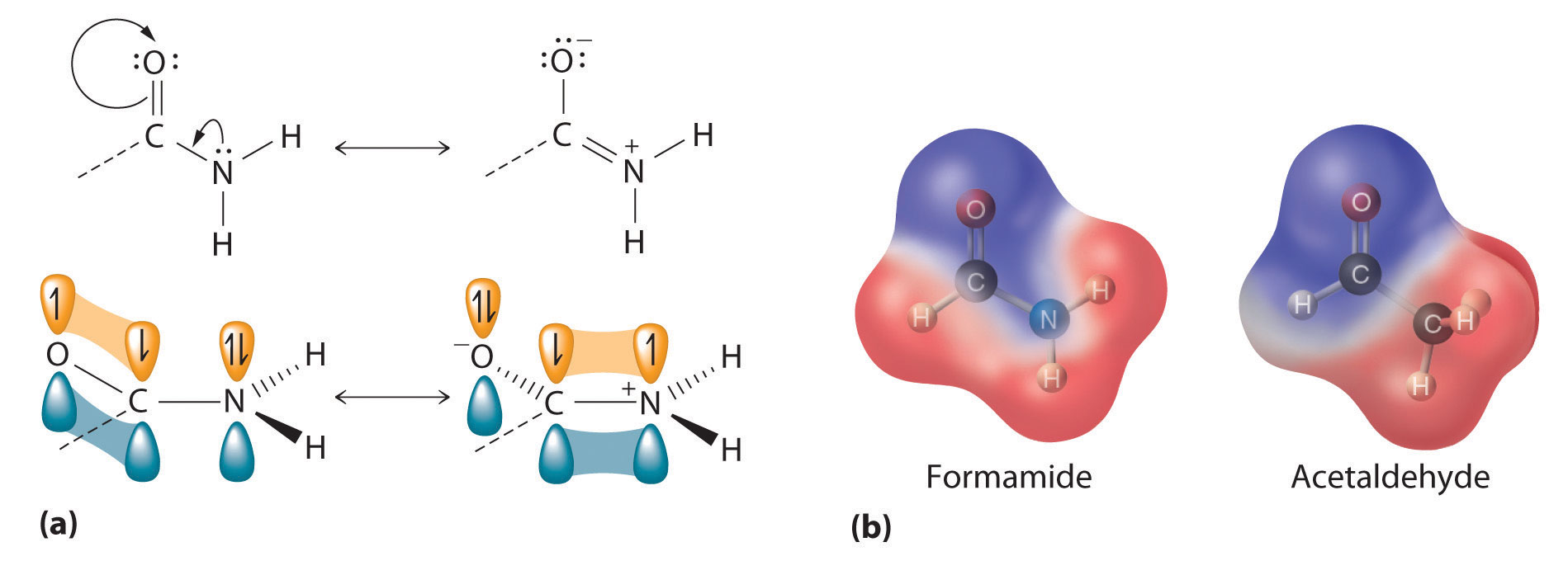
(a) An unhybridized 2pz orbital on nitrogen, containing a lone electron pair of electrons, can interact with the π orbital of the carbonyl group to give a three-center, four-electron bond. This interaction reduces the reactivity of the amide, making amides the least reactive of the carboxylic acid derivatives. (b) A comparison of the electrostatic potential maps of acetaldehyde and formamide shows that the negative charge (indicated in blue) is more localized on the oxygen atom of acetaldehyde than it is in formamide. Formamide is therefore less reactive.
Amines are derivatives of ammonia in which one or more hydrogen atoms have been replaced by alkyl or aryl groups. They are therefore analogous to alcohols and ethers. Like alcohols, amines are classified as primary, secondary, or tertiary, but in this case the designation refers to the number of alkyl groups bonded to the nitrogen atom, not to the number of adjacent carbon atoms. In primary amines, the nitrogen is bonded to two hydrogen atoms and one alkyl group; in secondary amines, the nitrogen is bonded to one hydrogen and two alkyl groups; and in tertiary amines, the nitrogen is bonded to three alkyl groups. With one lone pair of electrons and C–N bonds that are less polar than C–O bonds, ammonia and simple amines have much lower boiling points than water or alcohols with similar molecular masses. Primary amines tend to have boiling points intermediate between those of the corresponding alcohol and alkane. Moreover, secondary and tertiary amines have lower boiling points than primary amines of comparable molecular mass.
Tertiary amines form cations analogous to the ammonium ion (NH4+), in which all four H atoms are replaced by alkyl groups. Such substances, called quaternary ammonium saltsA salt that consist of an anion and a cation in which all four H atoms of the ammonium ion are replaced by alkyl groups., can be chiral if all four substituents are different. (Amines with three different substituents are also chiral because the lone pair of electrons represents a fourth substituent.)
Alkylamines can be prepared by nucleophilic substitution reactions of alkyl halides with ammonia or other amines:
Equation 24.12
RCl + NH3 → RNH2 + HClEquation 24.13
RCl + R′NH2 → RR′NH + HClEquation 24.14
RCl + R′R″NH → RR′R″N + HClThe primary amine formed in the first reaction (Equation 24.12) can react with more alkyl halide to generate a secondary amine (Equation 24.13), which in turn can react to form a tertiary amine (Equation 24.14). Consequently, the actual reaction mixture contains primary, secondary, and tertiary amines and even quaternary ammonium salts.
The reactions of amines are dominated by two properties: their ability to act as weak bases and their tendency to act as nucleophiles, both of which are due to the presence of the lone pair of electrons on the nitrogen atom. Amines typically behave as bases by accepting a proton from an acid to form an ammonium salt, as in the reaction of triethylamine (the ethyl group is represented as Et) with aqueous HCl (the lone pair of electrons on nitrogen is shown):
Equation 24.15
Et3N:(l) + HCl(aq) → Et3NH+Cl−(aq)which gives triethylammonium chloride. Amines can react with virtually any electrophile, including the carbonyl carbon of an aldehyde, a ketone, or an ester. Aryl amines such as aniline (C6H5NH2) are much weaker bases than alkylamines because the lone pair of electrons on nitrogen interacts with the π bonds of the aromatic ring, delocalizing the lone pair through resonance (Figure 24.20 "Structures and Basicity of Aniline and Cyclohexylamine").
The reactions of amines are dominated by their ability to act as weak bases and their tendency to act as nucleophiles.
Figure 24.20 Structures and Basicity of Aniline and Cyclohexylamine
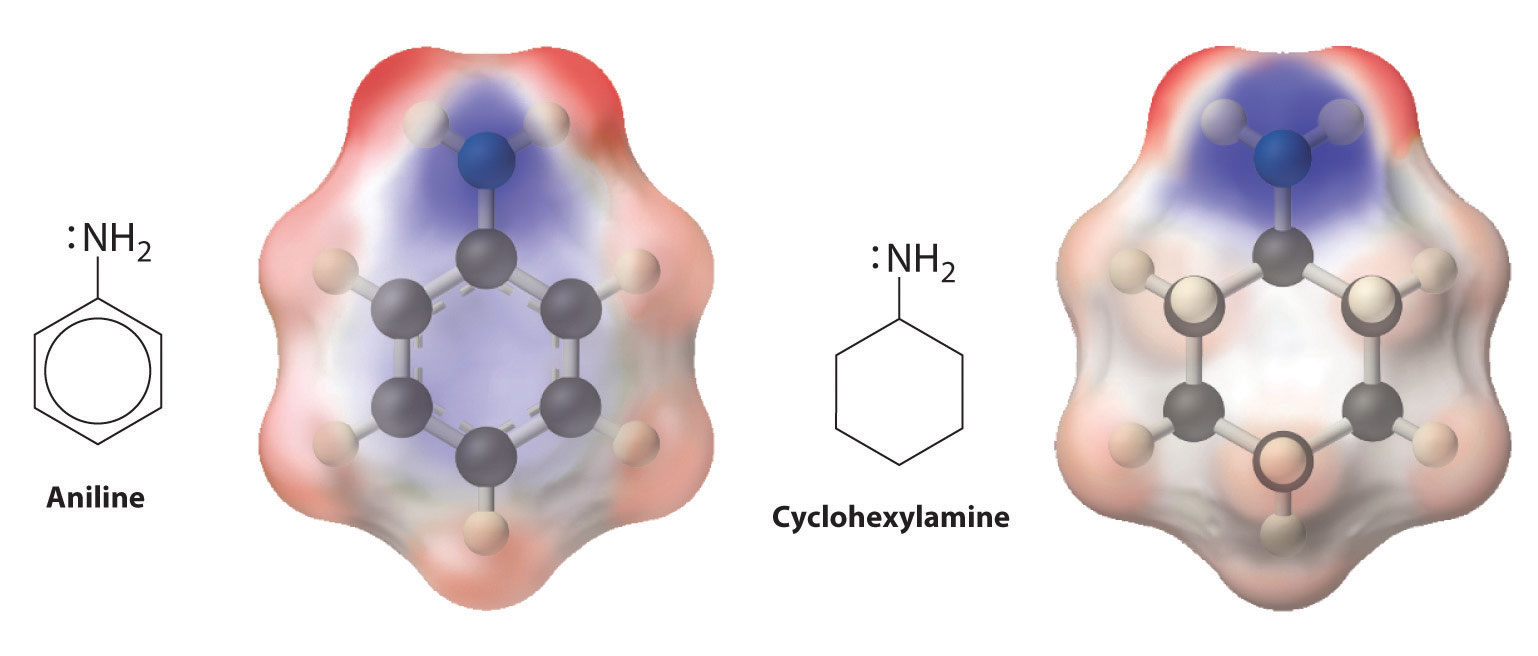
Delocalization of the lone electron pair on N over the benzene ring reduces the basicity of aryl amines, such as aniline, compared with that of alkylamines, such as cyclohexylamine. These electrostatic potential maps show that the electron density on the N of cyclohexylamine is more localized than it is in aniline, which makes cyclohexylamine a stronger base.
Predict the products formed in each reaction and show the initial site of attack and, for part (b), the final products.
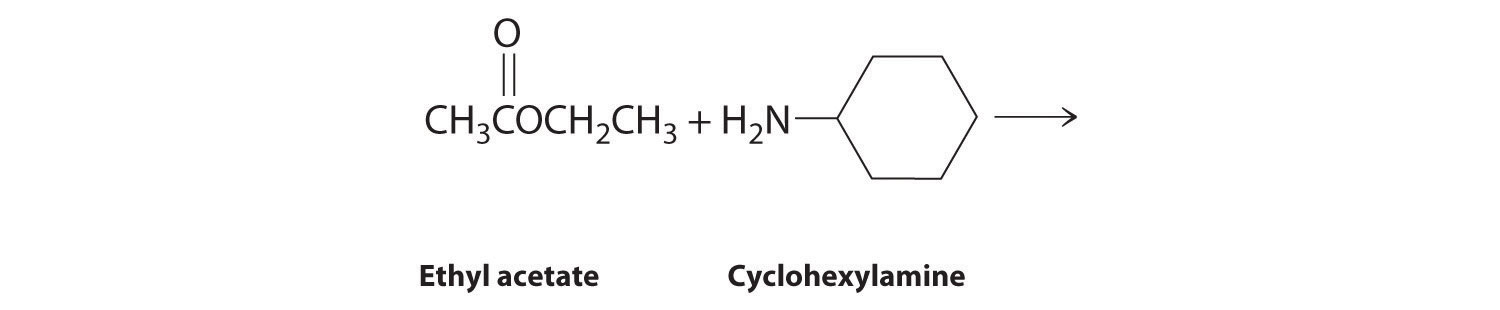
Given: reactants
Asked for: products and mechanism of reaction
Strategy:
Use the strategy outlined in Example 7.
Solution:
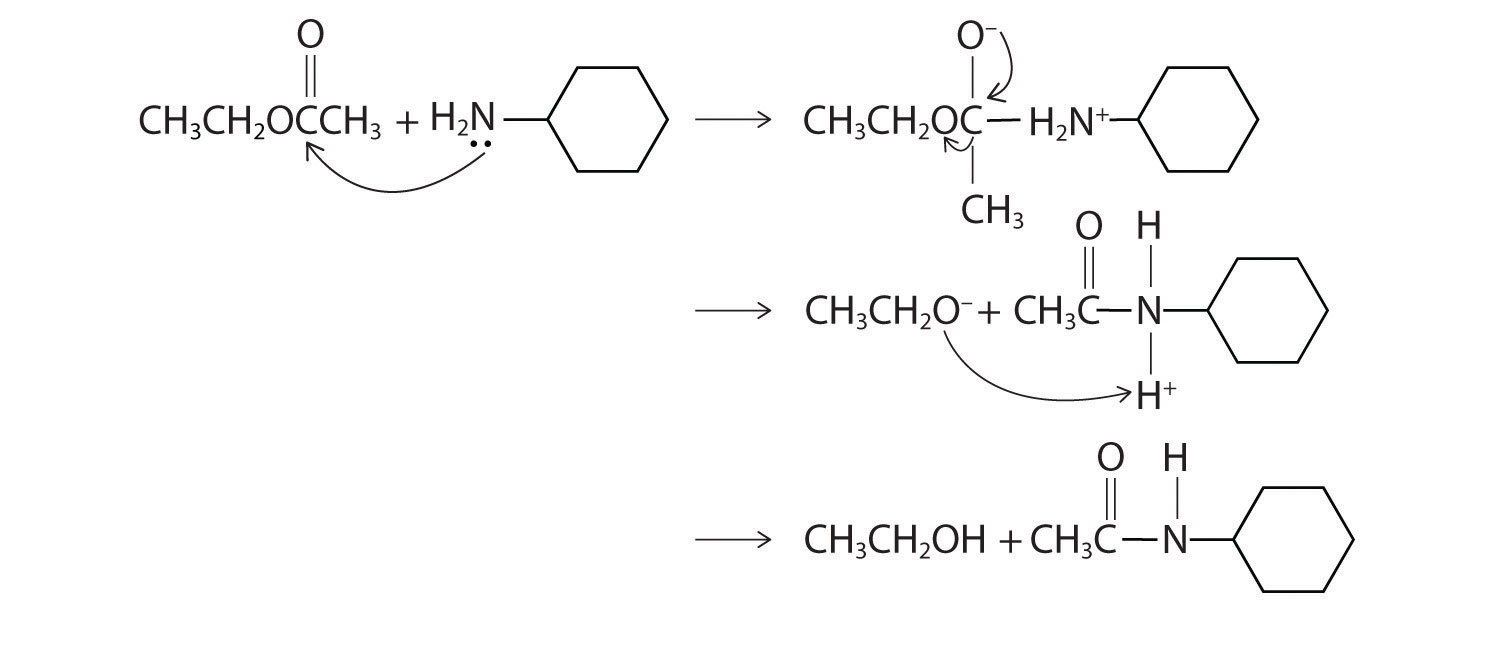
Exercise
Predict the products of each reaction. State the initial site of attack.
Answer:
Initial attack occurs with protonation of the oxygen of the carbonyl. The products are:
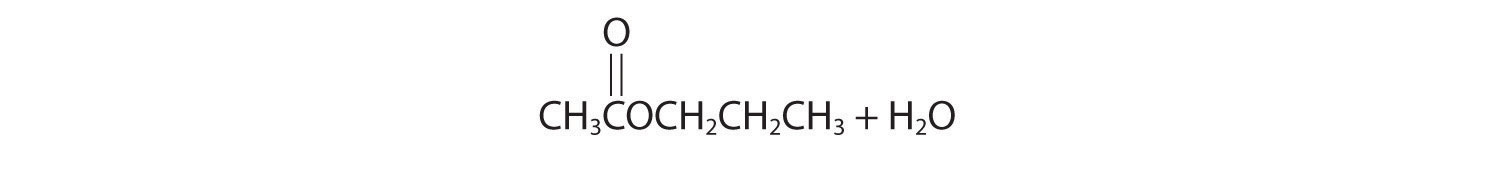
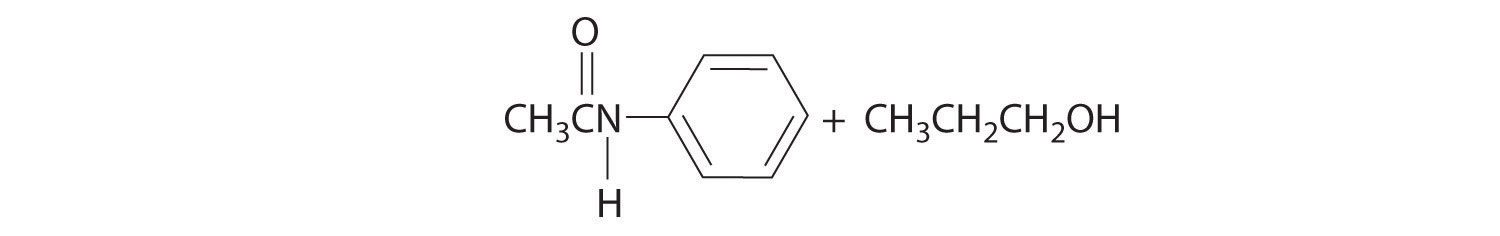
Initial attack occurs at the carbon of the carbonyl group. The products are:
Reactions like we have discussed in this section and Section 24.4 "Common Classes of Organic Reactions" are used to synthesize a wide range of organic compounds. When chemists plan the synthesis of an organic molecule, however, they must take into consideration various factors, such as the availability and cost of reactants, the need to minimize the formation of undesired products, and the proper sequencing of reactions to maximize the yield of the target molecule and minimize the formation of undesired products. Because the synthesis of many organic molecules requires multiple steps, in designing a synthetic scheme for such molecules, chemists must often work backward from the desired product in a process called retrosynthesis. Using this process, they can identify the reaction steps needed to synthesize the desired product from the available reactants.
There are strong connections among the structure, the physical properties, and the reactivity for compounds that contain the major functional groups. Hydrocarbons that are alkanes undergo catalytic cracking, which can convert straight-chain alkanes to highly branched alkanes. Catalytic cracking is one example of a pyrolysis reaction, in which the weakest bond is cleaved at high temperature, producing a mixture of radicals. The multiple bond of an alkene produces geometric isomers (cis and trans). Terminal alkynes contain a hydrogen atom directly attached to a triply bonded carbon. Removal of the hydrogen forms an acetylide ion, a potent nucleophile used to make longer carbon chains. Arenes undergo substitution rather than elimination because of enhanced stability from delocalization of their π electron density. An alcohol is often prepared by adding the elements of water across a double bond or by a substitution reaction. Alcohols undergo two major types of reactions: those involving cleavage of the O–H bond and those involving cleavage of the C–O bond. Phenols are acidic because of π interactions between the oxygen atom and the ring. Ethers are comparatively unreactive. Aldehydes and ketones are generally prepared by oxidizing alcohols. Their chemistry is characterized by nucleophilic attack at the carbon atom of the carbonyl functional group and electrophilic attack at the oxygen atom. Grignard reagents (RMgX, where X is Cl, Br, or I) convert the carbonyl functional group to an alcohol and lengthen the carbon chain. Compounds that contain the carboxyl functional group are weakly acidic because of delocalization of the π electrons, which causes them to easily lose a proton and form the carboxylate anion. Carboxylic acids are generally prepared by oxidizing alcohols and aldehydes or reacting a Grignard reagent with CO2. Carboxylic acid derivatives include esters, prepared by reacting a carboxylic acid and an alcohol, and amides, prepared by the nucleophilic reaction of amines with more electrophilic carboxylic acid derivatives, such as esters. Amides are relatively unreactive because of π bonding interactions between the lone pair on nitrogen and the carbonyl group. Amines can also be primary, secondary, or tertiary, depending on the number of alkyl groups bonded to the amine. Quaternary ammonium salts have four substituents attached to nitrogen and can be chiral. Amines are often prepared by a nucleophilic substitution reaction between a polar alkyl halide and ammonia or other amines. They are nucleophiles, but their base strength depends on their substituents.
Why do branched-chain alkanes have lower melting points than straight-chain alkanes of comparable molecular mass?
Describe alkanes in terms of their orbital hybridization, polarity, and reactivity. What is the geometry about each carbon of a straight-chain alkane?
Why do alkenes form cis and trans isomers, whereas alkanes do not? Do alkynes form cis and trans isomers? Why or why not?
Which compounds can exist as cis and trans isomers?
Which compounds can exist as cis and trans isomers?
Which compounds have a net dipole moment?
Why is the boiling point of an alcohol so much greater than that of an alkane of comparable molecular mass? Why are low-molecular-mass alcohols reasonably good solvents for some ionic compounds, whereas alkanes are not?
Is an alcohol a nucleophile or an electrophile? What determines the mode of reactivity of an alcohol? How does the reactivity of an alcohol differ from that of an ionic compound containing OH, such as KOH?
How does the reactivity of ethers compare with that of alcohols? Why? Ethers can be cleaved under strongly acidic conditions. Explain how this can occur.
What functional group is common to aldehydes, ketones, carboxylic acids, and esters? This functional group can react with both nucleophiles and electrophiles. Where does nucleophilic attack on this functional group occur? Where does electrophilic attack occur?
What key feature of a Grignard reagent allows it to engage in a nucleophilic attack on a carbonyl carbon?
Do you expect carboxylic acids to be more or less water soluble than ketones of comparable molecular mass? Why?
Because amides are formally derived from an acid plus an amine, why can they not be prepared by the reaction of an acid with an amine? How are they generally prepared?
Is an amide susceptible to nucleophilic attack, electrophilic attack, or both? Specify where the attack occurs.
What factors determine the reactivity of amines?
(c) and (d)
The presence of a nucleophilic Cδ− resulting from a highly polar interaction with an electropositive Mg
Their ability to act as weak bases and their tendency to act as nucleophiles
What is the product of the reaction of 2-butyne with excess HBr?
What is the product of the reaction of 3-hexyne with excess HCl?
What elements are eliminated during the dehydrohalogenation of an alkyl halide? What products do you expect from the dehydrohalogenation of 2-chloro-1-pentene?
What elements are eliminated during the dehydration of an alcohol? What products do you expect from the dehydration of ethanol?
Predict the products of each reaction.
Show the mechanism and predict the organic product of each reaction.
A Grignard reagent can be used to generate a carboxylic acid. Show the mechanism for the first step in this reaction using CH3CH2MgBr as the Grignard reagent. What is the geometry about the carbon of the –CH2 of the intermediate species formed in this first step?
Draw a molecular orbital picture showing the bonding in an amide. What orbital is used for the lone pair of electrons on nitrogen?
What is the product of the reaction of
Develop a synthetic scheme to generate
2,2-dibromobutane