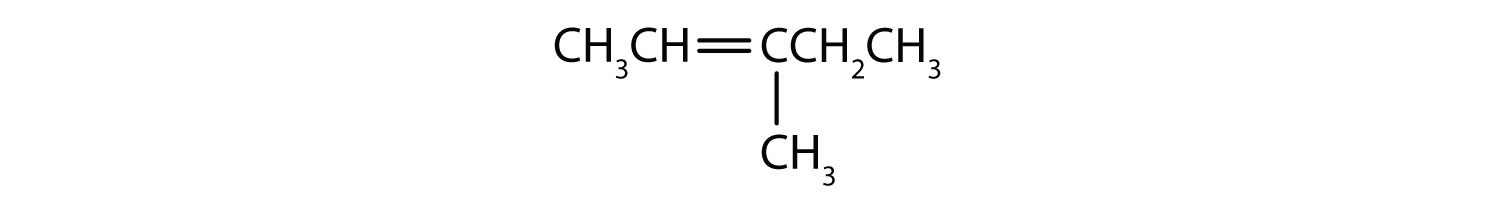To ensure that you understand the material in this chapter, you should review the meanings of the bold terms in the following summary and ask yourself how they relate to the topics in the chapter.
Any hydrocarbon containing either a double or triple bond is an unsaturated hydrocarbon. Alkenes have a carbon-to-carbon double bond. The general formula for alkenes with one double bond is CnH2n. Alkenes can be straight chain, branched chain, or cyclic. Simple alkenes often have common names, but all alkenes can be named by the system of the International Union of Pure and Applied Chemistry.
Cis-trans isomers (or geometric isomers) are characterized by molecules that differ only in their configuration around a rigid part of the structure, such as a carbon–to-carbon double bond or a ring. The molecule having two identical (or closely related) atoms or groups on the same side is the cis isomer; the one having the two groups on opposite sides is the trans isomer.
The physical properties of alkenes are quite similar to those of alkanes. Like other hydrocarbons, alkenes are insoluble in water but soluble in organic solvents.
More reactive than alkanes, alkenes undergo addition reactions across the double bond:
Addition of hydrogen (hydrogenation):
CH2=CH2 + H2 → CH3CH3Addition of halogen (halogenation):
CH2=CH2 + X2 → XCH2CH2Xwhere X = F, Cl, Br, or I.
Addition of water (hydration):
CH2=CH2 + HOH → HCH2CH2OHAlkenes also undergo addition polymerization, molecules joining together to form long-chain molecules.
…CH2=CH2 + CH2=CH2 + CH2=CH2 +…→…CH2CH2–CH2CH2–CH2CH2–…The reactant units are monomers, and the product is a polymer.
Alkynes have a carbon-to-carbon triple bond. The general formula for alkynes is CnH2n − 2. The properties of alkynes are quite similar to those of alkenes. They are named much like alkenes but with the ending -yne.
The cyclic hydrocarbon benzene (C6H6) has a ring of carbon atoms. The molecule seems to be unsaturated, but it does not undergo the typical reactions expected of alkenes. The electrons that might be fixed in three double bonds are instead delocalized over all six carbon atoms.
A hydrocarbon containing one or more benzene rings (or other similarly stable electron arrangements) is an aromatic hydrocarbon, and any related substance is an aromatic compound. One or more of the hydrogen atoms on a benzene ring can be replaced by other atoms. When two hydrogen atoms are replaced, the product name is based on the relative position of the replacement atoms (or atom groups). A 1,2-disubstituted benzene is designated as an ortho (o-) isomer; 1,3-, a meta (m-) isomer; and 1,4-, a para (p-) isomer. An aromatic group as a substituent is called an aryl group.
A polycyclic aromatic hydrocarbon (PAH) has fused benzene rings sharing a common side.
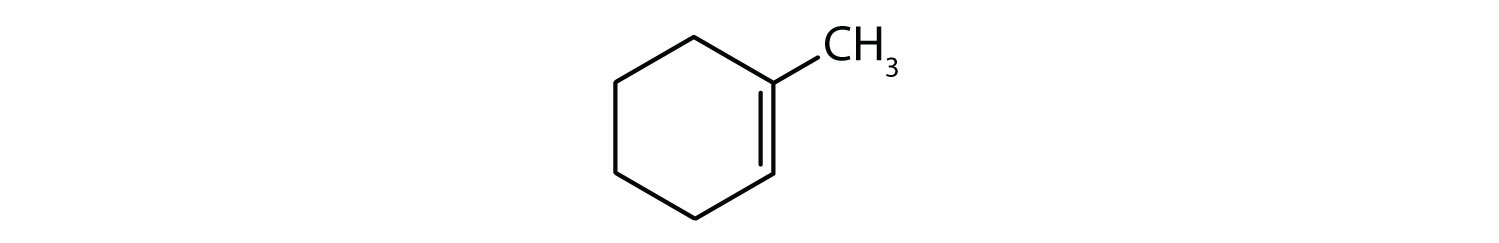
Classify each compound as saturated or unsaturated.
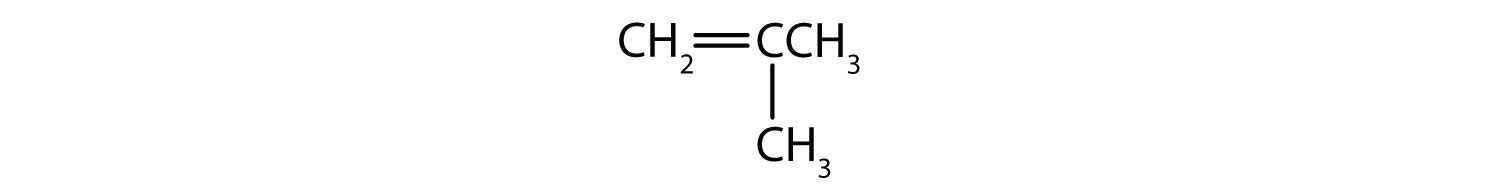
Classify each compound as saturated or unsaturated.
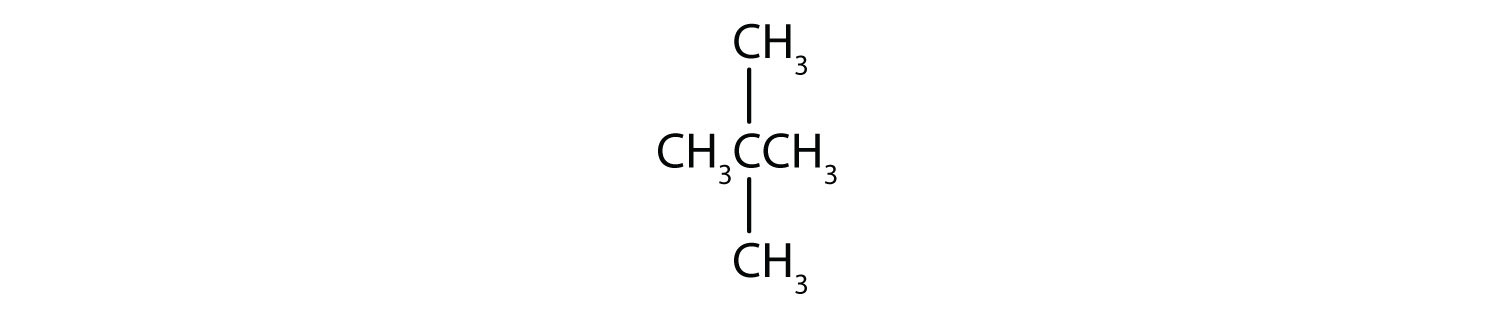
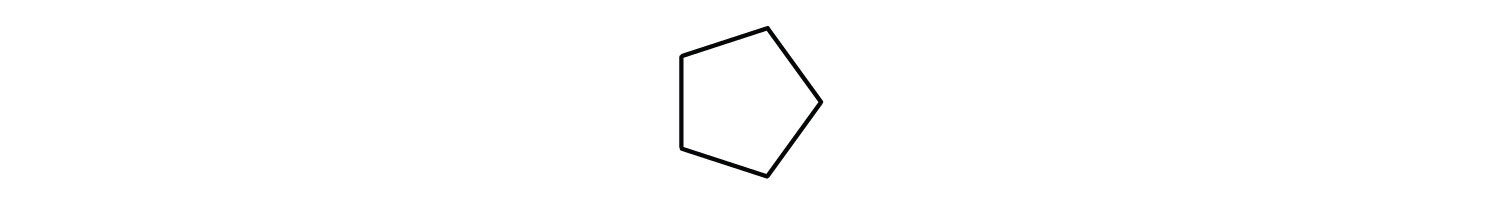
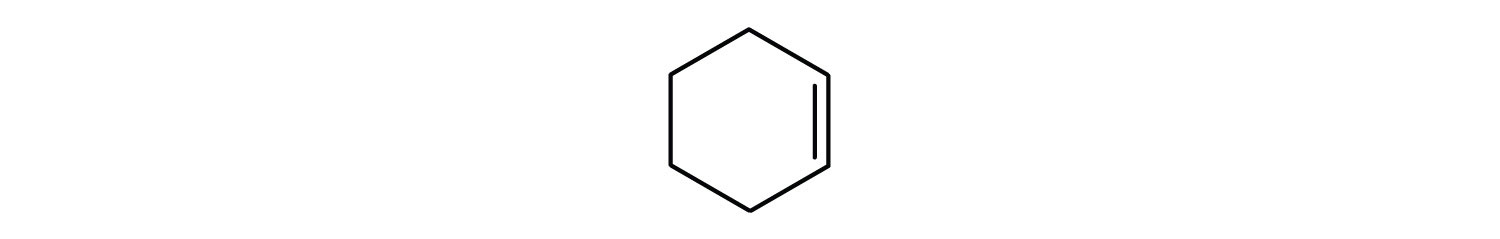
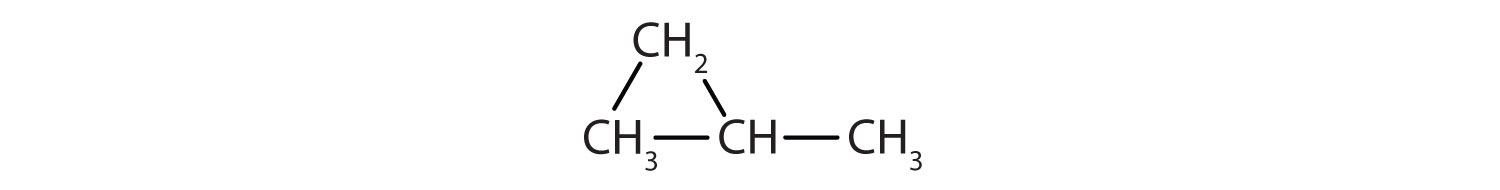
Give the molecular formula for each compound.
When three isomeric pentenes—X, Y, and Z—are hydrogenated, all three form 2-methylbutane. The addition of Cl2 to Y gives 1,2-dichloro-3-methylbutane, and the addition of Cl2 to Z gives 1,2-dichloro-2-methylbutane. Draw the original structures for X, Y, and Z.
Pentane and 1-pentene are both colorless, low-boiling liquids. Describe a simple test that distinguishes the two compounds. Indicate what you would observe.
Draw and name all the alkene cis-trans isomers corresponding to the molecular formula C5H10. (Hint: there are only two.)
The complete combustion of benzene forms carbon dioxide and water:
C6H6 + O2 → CO2 + H2OBalance the equation. What mass, in grams, of carbon dioxide is formed by the complete combustion of 39.0 g of benzene?
Describe a physiological effect of some PAHs.
What are some of the hazards associated with the use of benzene?
What is wrong with each name? Draw the structure and give the correct name for each compound.
What is wrong with each name?
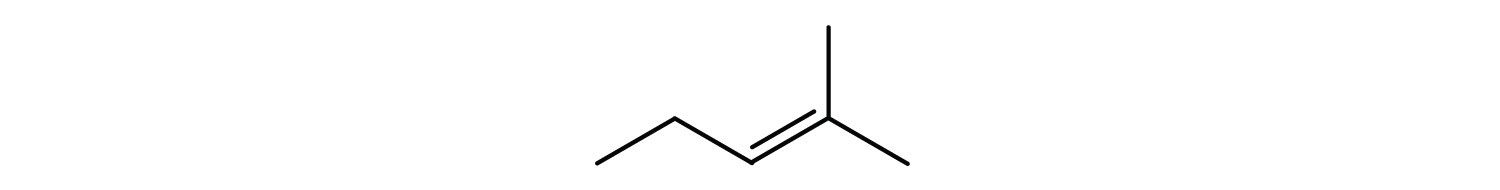
Following are line-angle formulas for three compounds. Draw the structure and give the name for each.
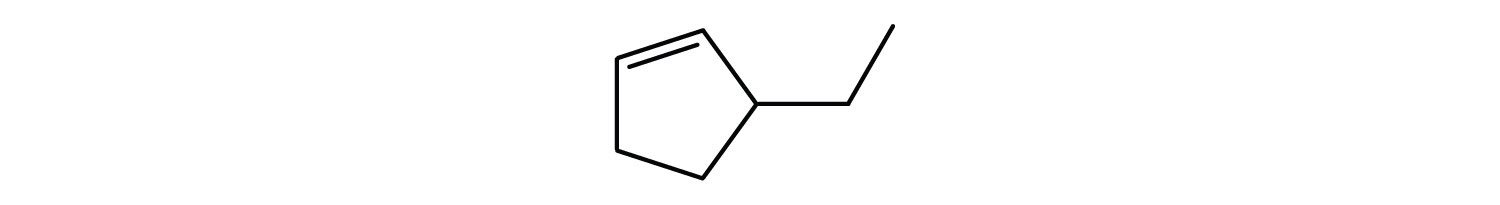
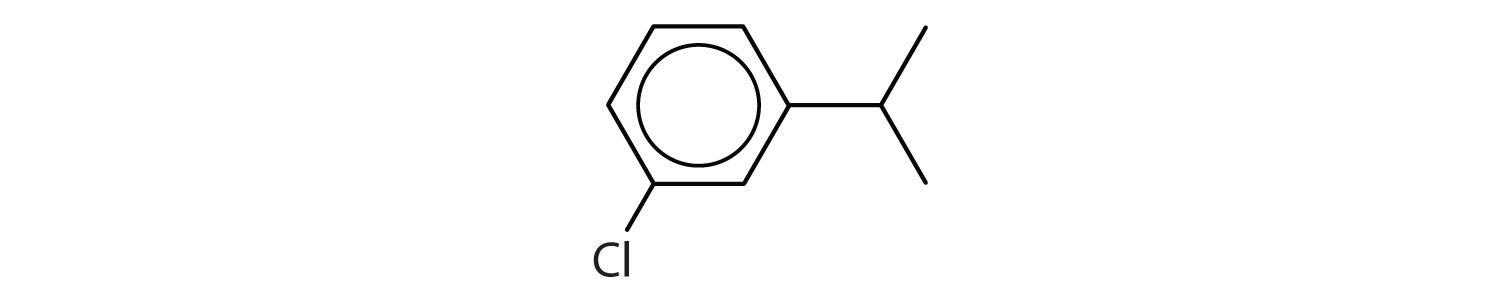
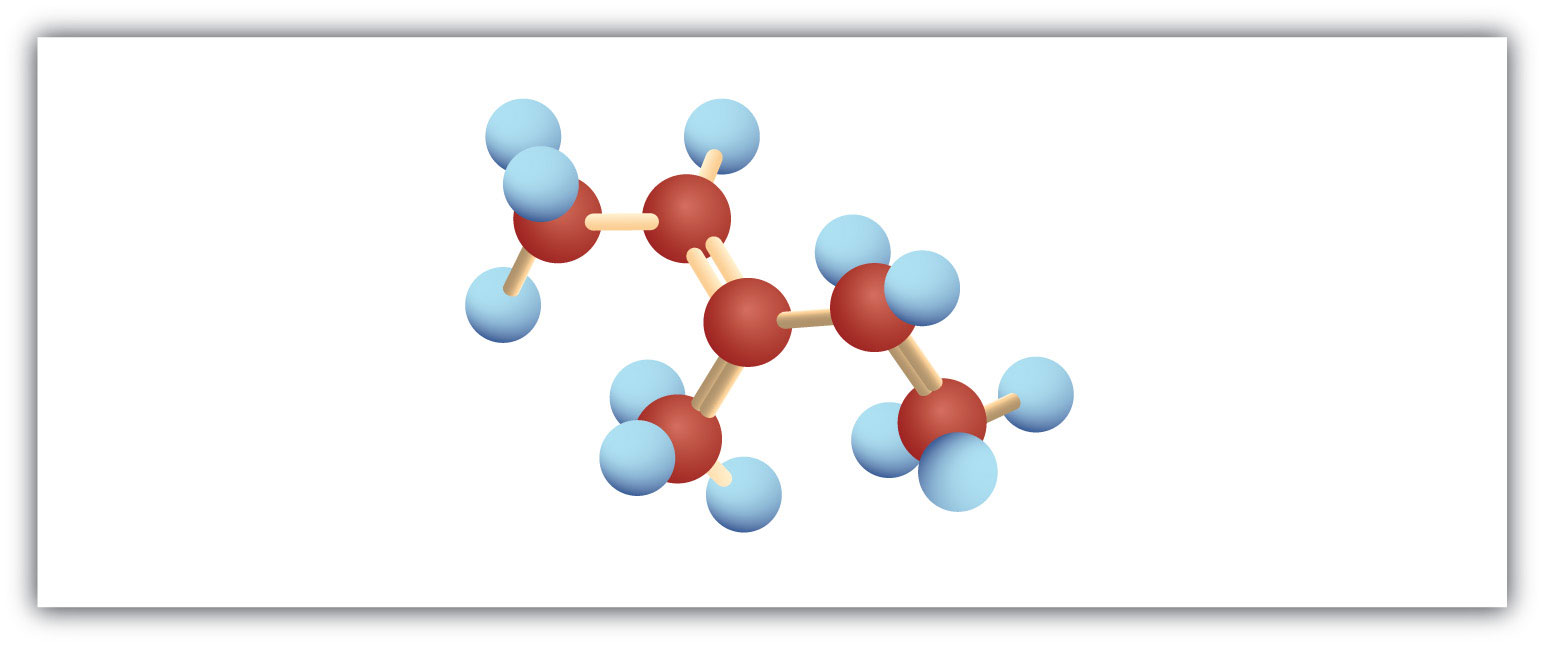
Following are ball-and-stick molecular models for three compounds (blue balls represent H atoms; red balls are C atoms). Write the condensed structural formula and give the name for each.
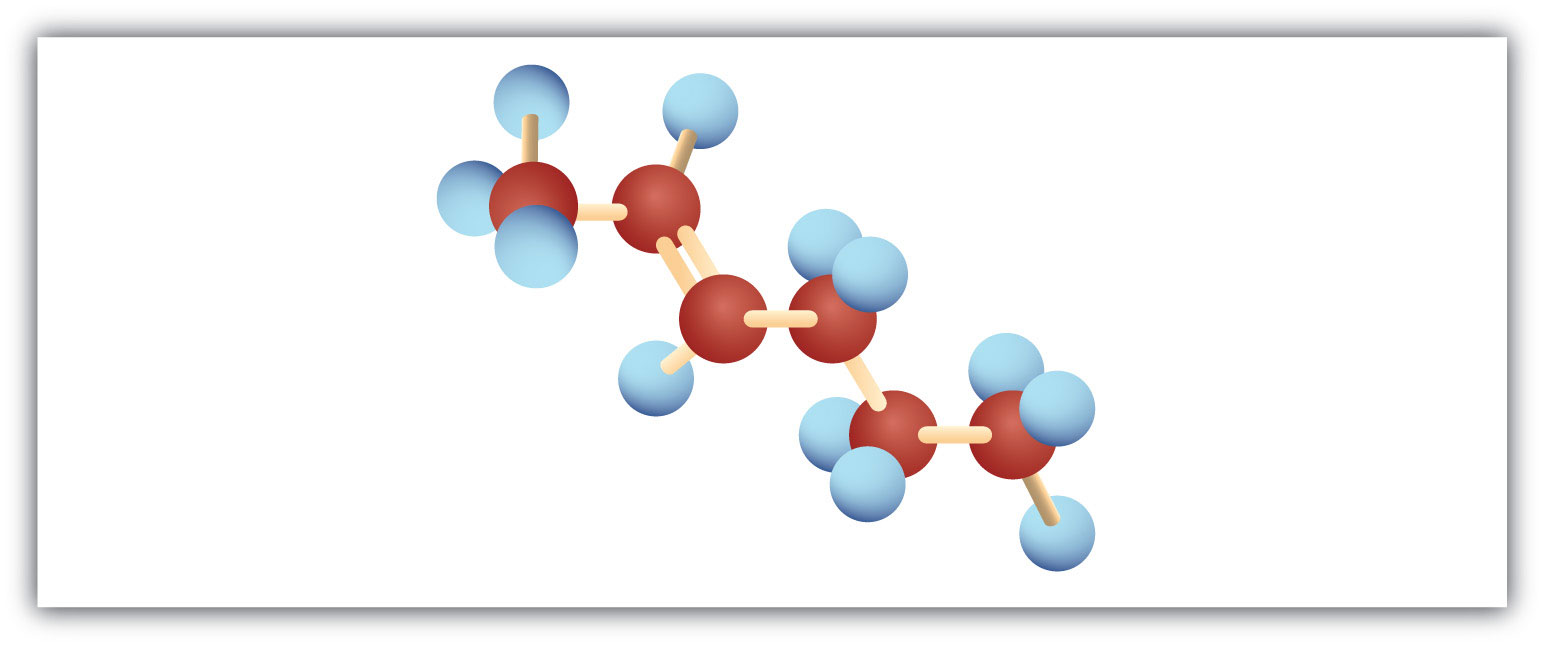
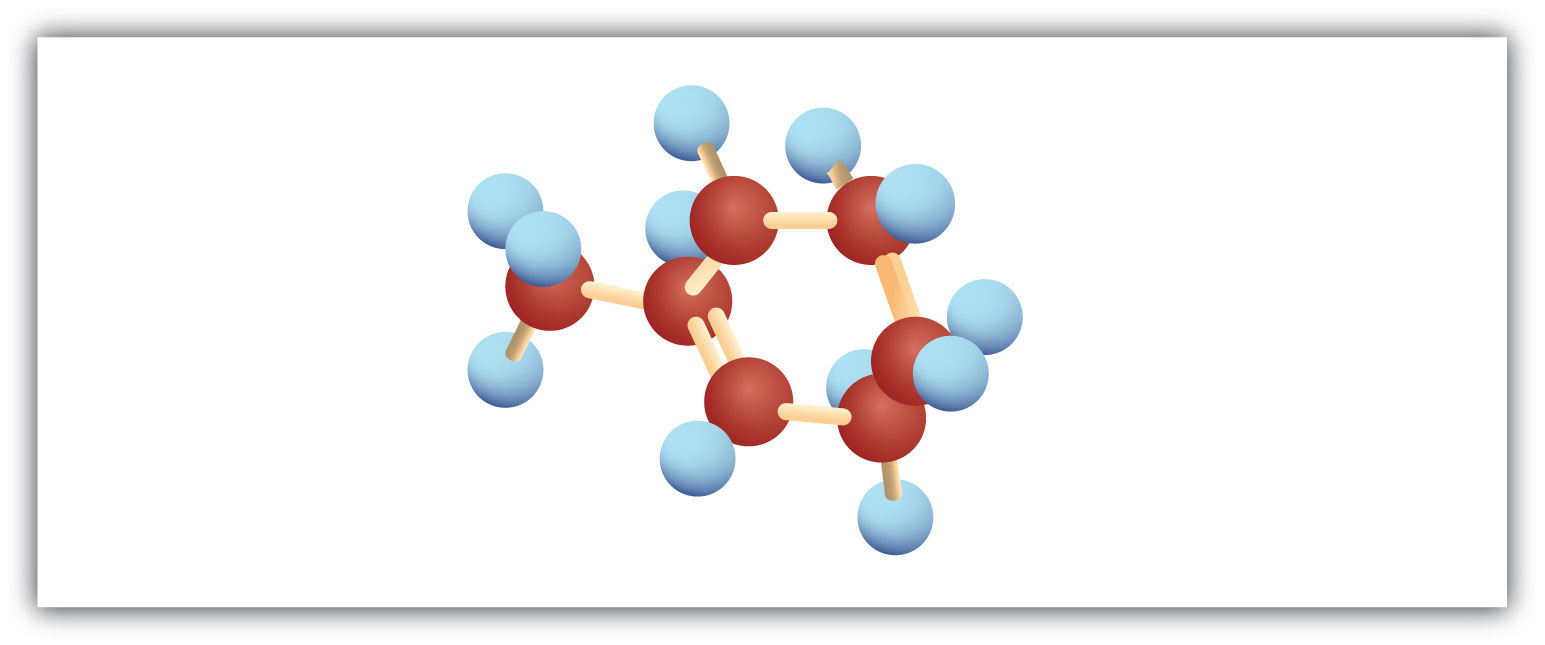
Add bromine solution (reddish-brown) to each. Pentane will not react, and the reddish-brown color persists; 1-pentene will react, leaving a colorless solution.
2C6H6 + 15O2 → 12CO2 + 6H2O; 132 g
carcinogenic, flammable