More than 70% of the earth’s surface is covered by a very important solution—seawater. It is likely that without seawater, no life would exist on Earth.
At its simplest, seawater is mostly H2O. But about 3.5% of seawater is dissolved solids, mostly NaCl but other ions as well. Table 11.1 "Percentage by Mass of Ions in Seawater and Blood" lists the percentage by mass of the various ions in seawater.
Because it is highly likely that life on Earth originated in the oceans, it should not be surprising that many bodily fluids resemble seawater—especially blood. Table 11.1 "Percentage by Mass of Ions in Seawater and Blood" also lists the percentage by mass of ions in a typical sample of blood.
Table 11.1 Percentage by Mass of Ions in Seawater and Blood
| Ion | Percentage in Seawater | Percentage in Blood |
|---|---|---|
| Na+ | 2.36 | 0.322 |
| Cl− | 1.94 | 0.366 |
| Mg2+ | 0.13 | 0.002 |
| SO42− | 0.09 | — |
| K+ | 0.04 | 0.016 |
| Ca2+ | 0.04 | 0.0096 |
| HCO3− | 0.002 | 0.165 |
| HPO42−, H2PO4− | — | 0.01 |
Most ions are more abundant in seawater than they are in blood, with some notable exceptions. There is far more hydrogen carbonate ion (HCO3−) in blood than in seawater; indeed, it is the third most common ion in blood. This difference is significant because the HCO3− ion and some related species [CO32−, CO2(aq)] have an important role in controlling the acid-base properties of blood. Although there is a negligible amount of the two hydrogen phosphate ions (HPO42− and H2PO4−) in seawater, there is a small amount in blood, where these ions affect acid-base properties. Another notable difference is that blood has a negligible amount of the sulfate ion (SO42−), but this ion is present in seawater.
Gold is present in seawater—but only a tiny amount. A current estimate of the amount of gold is about 1 part per every 1 × 1013 parts of seawater, which makes the extraction of gold from seawater unfeasible. However, it does mean that there are about 1.4 × 1014 g of gold in the world’s oceans!
A solution is a homogeneous mixture—a mixture of two or more substances that are so intimately mixed that the mixture behaves in many ways like a single substance. Many chemical reactions occur when the reactants are dissolved in solution. In this chapter, we will introduce concepts that are applicable to solutions and the chemical reactions that occur in them.
The major component of a solution is called the solventThe major component of a solution.. The minor component of a solution is called the soluteThe minor component of a solution.. By major and minor we mean whichever component has the greater presence by mass or by moles. Sometimes this becomes confusing, especially with substances with very different molar masses. However, here we will confine the discussion to solutions for which the major component and the minor component are obvious.
Solutions exist for every possible phase of the solute and the solvent. Salt water, for example, is a solution of solid NaCl in liquid water; soda water is a solution of gaseous CO2 in liquid water, while air is a solution of a gaseous solute (O2) in a gaseous solvent (N2). In all cases, however, the overall phase of the solution is the same phase as the solvent.
A solution is made by dissolving 1.00 g of sucrose (C12H22O11) in 100.0 g of liquid water. Identify the solvent and solute in the resulting solution.
Solution
Either by mass or by moles, the obvious minor component is sucrose, so it is the solute. Water—the majority component—is the solvent. The fact that the resulting solution is the same phase as water also suggests that water is the solvent.
Test Yourself
A solution is made by dissolving 3.33 g of HCl(g) in 40.0 g of liquid methyl alcohol (CH3OH). Identify the solvent and solute in the resulting solution.
Answer
solute: HCl(g); solvent: CH3OH
One important concept of solutions is in defining how much solute is dissolved in a given amount of solvent. This concept is called concentrationHow much solute is dissolved in a given amount of solvent.. Various words are used to describe the relative amounts of solute. DiluteA solution with very little solute. describes a solution that has very little solute, while concentratedA solution with a lot of solute. describes a solution that has a lot of solute. One problem is that these terms are qualitative; they describe more or less but not exactly how much.
In most cases, only a certain maximum amount of solute can be dissolved in a given amount of solvent. This maximum amount is called the solubilityThe maximum amount of a solute that can be dissolved in a given amount of a solvent. of the solute. It is usually expressed in terms of the amount of solute that can dissolve in 100 g of the solvent at a given temperature. Table 11.2 "Solubilities of Some Ionic Compounds" lists the solubilities of some simple ionic compounds. These solubilities vary widely: NaCl can dissolve up to 31.6 g per 100 g of H2O, while AgCl can dissolve only 0.00019 g per 100 g of H2O.
Table 11.2 Solubilities of Some Ionic Compounds
| Solute | Solubility (g per 100 g of H2O at 25°C) |
|---|---|
| AgCl | 0.00019 |
| CaCO3 | 0.0006 |
| KBr | 70.7 |
| NaCl | 36.1 |
| NaNO3 | 94.6 |
When the maximum amount of solute has been dissolved in a given amount of solvent, we say that the solution is saturatedA solution with the maximum amount of solute dissolved in it. with solute. When less than the maximum amount of solute is dissolved in a given amount of solute, the solution is unsaturatedA solution with less than the maximum amount of solute dissolved in it.. These terms are also qualitative terms because each solute has its own solubility. A solution of 0.00019 g of AgCl per 100 g of H2O may be saturated, but with so little solute dissolved, it is also rather dilute. A solution of 36.1 g of NaCl in 100 g of H2O is also saturated but rather concentrated. Ideally, we need more precise ways of specifying the amount of solute in a solution. We will introduce such ways in Section 11.2 "Quantitative Units of Concentration".
In some circumstances, it is possible to dissolve more than the maximum amount of a solute in a solution. Usually, this happens by heating the solvent, dissolving more solute than would normally dissolve at regular temperatures, and letting the solution cool down slowly and carefully. Such solutions are called supersaturatedA unstable solution with more than the normal maximum amount of solute in it. solutions and are not stable; given an opportunity (such as dropping a crystal of solute in the solution), the excess solute will precipitate from the solution.
It should be obvious that some solutes dissolve in certain solvents but not others. NaCl, for example, dissolves in water but not in vegetable oil. Beeswax dissolves in liquid hexane but not water. What is it that makes a solute soluble in some solvents but not others?
The answer is intermolecular interactions. The intermolecular interactions include London dispersion forces, dipole-dipole interactions, and hydrogen bonding (as described in Chapter 10 "Solids and Liquids"). From experimental studies, it has been determined that if molecules of a solute experience the same intermolecular forces that the solvent does, the solute will likely dissolve in that solvent. So, NaCl—a very polar substance because it is composed of ions—dissolves in water, which is very polar, but not in oil, which is generally nonpolar. Nonpolar wax dissolves in nonpolar hexane but not in polar water. This concept leads to the general rule that “like dissolves like” for predicting whether a solute is soluble in a given solvent. However, this is a general rule, not an absolute statement, so it must be applied with care.
Would I2 be more soluble in CCl4 or H2O? Explain your answer.
Solution
I2 is nonpolar. Of the two solvents, CCl4 is nonpolar and H2O is polar, so I2 would be expected to be more soluble in CCl4.
Test Yourself
Would C3H7OH be more soluble in CCl4 or H2O? Explain your answer.
Answer
H2O because both experience hydrogen bonding
Define solute and solvent.
Define saturated, unsaturated, and supersaturated.
A solution is prepared by combining 2.09 g of CO2 and 35.5 g of H2O. Identify the solute and solvent.
A solution is prepared by combining 10.3 g of Hg(ℓ) and 45.0 g of Ag(s). Identify the solute and solvent.
Use Table 11.2 "Solubilities of Some Ionic Compounds" to decide if a solution containing 45.0 g of NaCl per 100 g of H2O is unsaturated, saturated, or supersaturated.
Use Table 11.2 "Solubilities of Some Ionic Compounds" to decide if a solution containing 0.000092 g of AgCl per 100 g of H2O is unsaturated, saturated, or supersaturated.
Would the solution in Exercise 5 be described as dilute or concentrated? Explain your answer.
Would the solution in Exercise 6 be described as dilute or concentrated? Explain your answer.
Identify a solute from Table 11.2 "Solubilities of Some Ionic Compounds" whose saturated solution can be described as dilute.
Identify a solute from Table 11.2 "Solubilities of Some Ionic Compounds" whose saturated solution can be described as concentrated.
Which solvent is Br2 more likely soluble in—CH3OH or C6H6?
Which solvent is NaOH more likely soluble in—CH3OH or C6H6?
Compounds with the formula CnH2n + 1OH are soluble in H2O when n is small but not when n is large. Suggest an explanation for this phenomenon.
Glucose has the following structure:
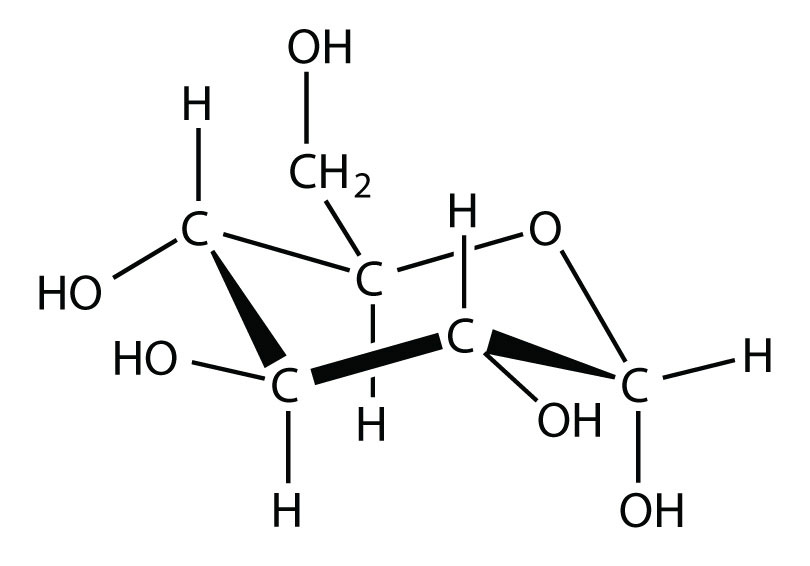
What parts of the molecule indicate that this substance is soluble in water?
The solvent is the majority component of a solution, whereas the solute is the minority component of a solution.
solute: CO2; solvent: H2O
supersaturated
concentrated because there is a lot of solute
AgCl or CaCO3
C6H6
The nonpolar end dominates intermolecular forces when n is large.
Rather than qualitative terms (Section 11.1 "Some Definitions"), we need quantitative ways to express the amount of solute in a solution; that is, we need specific units of concentration. In this section, we will introduce several common and useful units of concentration.
MolarityThe number of moles of solute divided by the number of liters of solution. (M) is defined as the number of moles of solute divided by the number of liters of solution:
which can be simplified as
As with any mathematical equation, if you know any two quantities, you can calculate the third, unknown, quantity.
For example, suppose you have 0.500 L of solution that has 0.24 mol of NaOH dissolved in it. The concentration of the solution can be calculated as follows:
The concentration of the solution is 0.48 M, which is spoken as “zero point forty-eight molarity” or “zero point forty-eight molar.” If the quantity of the solute is given in mass units, you must convert mass units to mole units before using the definition of molarity to calculate concentration. For example, what is the molar concentration of a solution of 22.4 g of HCl dissolved in 1.56 L? First, convert the mass of solute to moles using the molar mass of HCl (36.5 g/mol):
Now we can use the definition of molarity to determine a concentration:
What is the molarity of a solution made when 32.7 g of NaOH are dissolved to make 445 mL of solution?
Solution
To use the definition of molarity, both quantities must be converted to the proper units. First, convert the volume units from milliliters to liters:
Now we convert the amount of solute to moles, using the molar mass of NaOH, which is 40.0 g/mol:
Now we can use the definition of molarity to determine the molar concentration:
Test Yourself
What is the molarity of a solution made when 66.2 g of C6H12O6 are dissolved to make 235 mL of solution?
Answer
1.57 M
The definition of molarity can be used to determine the amount of solute or the volume of solution, if the other information is given. Example 4 illustrates this situation.
How many moles of solute are present in 0.108 L of a 0.887 M NaCl solution?
Solution
We know the volume and the molarity; we can use the definition of molarity to mathematically solve for the amount in moles. Substituting the quantities into the definition of molarity:
We multiply the 0.108 L over to the other side of the equation and multiply the units together; “molarity × liters” equals moles, according to the definition of molarity. So
mol NaCl = (0.887 M)(0.108 L) = 0.0958 molTest Yourself
How many moles of solute are present in 225 mL of a 1.44 M CaCl2 solution?
Answer
0.324 mol
If you need to determine volume, remember the rule that the unknown quantity must be by itself and in the numerator to determine the correct answer. Thus rearrangement of the definition of molarity is required.
What volume of a 2.33 M NaNO3 solution is needed to obtain 0.222 mol of solute?
Solution
Using the definition of molarity, we have
To solve for the number of liters, we bring the 2.33 M over to the right into the denominator, and the number of liters over to the left in the numerator. We now have
Dividing, the volume is 0.0953 L = 95.3 mL.
Test Yourself
What volume of a 0.570 M K2SO4 solution is needed to obtain 0.872 mol of solute?
Answer
1.53 L
A similar unit of concentration is molalityThe number of moles of solute per kilogram of solvent. (m), which is defined as the number of moles of solute per kilogram of solvent, not per liter of solution:
Mathematical manipulation of molality is the same as with molarity.
Another way to specify an amount is percentage composition by massRatio of mass of solute to the total mass of a sample times 100. (or mass percentage, % m/m). It is defined as follows:
It is not uncommon to see this unit used on commercial products (Figure 11.1 "Concentration in Commercial Applications").
What is the mass percentage of Fe in a piece of metal with 87.9 g of Fe in a 113 g sample?
Solution
Using the definition of mass percentage, we have
Test Yourself
What is the mass percentage of H2O2 in a solution with 1.67 g of H2O2 in a 55.5 g sample?
Answer
3.01%
Related concentration units are parts per thousand (ppth)Ratio of mass of solute to total mass of sample times 1,000., parts per million (ppm)Ratio of mass of solute to total mass of sample times 1,000,000., and parts per billion (ppb)Ratio of mass of solute to total mass of sample times 1,000,000,000.. Parts per thousand is defined as follows:
There are similar definitions for parts per million and parts per billion:
andEach unit is used for progressively lower and lower concentrations. The two masses must be expressed in the same unit of mass, so conversions may be necessary.
If there is 0.6 g of Pb present in 277 g of solution, what is the Pb concentration in parts per thousand?
Solution
Use the definition of parts per thousand to determine the concentration. Substituting
Test Yourself
If there is 0.551 mg of As in 348 g of solution, what is the As concentration in ppm?
Answer
1.58 ppm
As with molarity and molality, algebraic rearrangements may be necessary to answer certain questions.
The concentration of Cl– ion in a sample of H2O is 15.0 ppm. What mass of Cl– ion is present in 240.0 mL of H2O, which has a density of 1.00 g/mL?
Solution
First, use the density of H2O to determine the mass of the sample:
Now we can use the definition of ppm:
Rearranging to solve for the mass of solute,
Test Yourself
The concentration of Fe3+ ion in a sample of H2O is 335.0 ppm. What mass of Fe3+ ion is present in 3,450 mL of H2O, which has a density of 1.00 g/mL?
Answer
1.16 g
For ionic solutions, we need to differentiate between the concentration of the salt versus the concentration of each individual ion. Because the ions in ionic compounds go their own way when a compound is dissolved in a solution, the resulting concentration of the ion may be different from the concentration of the complete salt. For example, if 1 M NaCl were prepared, the solution could also be described as a solution of 1 M Na+(aq) and 1 M Cl−(aq) because there is one Na+ ion and one Cl− ion per formula unit of the salt. However, if the solution were 1 M CaCl2, there are two Cl−(aq) ions for every formula unit dissolved, so the concentration of Cl−(aq) would be 2 M, not 1 M.
In addition, the total ion concentration is the sum of the individual ion concentrations. Thus for the 1 M NaCl, the total ion concentration is 2 M; for the 1 M CaCl2, the total ion concentration is 3 M.
Differentiate between molarity and molality.
Differentiate between mass percentage and parts per thousand.
What is the molarity of a solution made by dissolving 13.4 g of NaNO3 in 345 mL of solution?
What is the molarity of a solution made by dissolving 332 g of C6H12O6 in 4.66 L of solution?
How many moles of MgCl2 are present in 0.0331 L of a 2.55 M solution?
How many moles of NH4Br are present in 88.9 mL of a 0.228 M solution?
What volume of 0.556 M NaCl is needed to obtain 0.882 mol of NaCl?
What volume of 3.99 M H2SO4 is needed to obtain 4.61 mol of H2SO4?
What volume of 0.333 M Al(NO3)3 is needed to obtain 26.7 g of Al(NO3)3?
What volume of 1.772 M BaCl2 is needed to obtain 123 g of BaCl2?
What are the individual ion concentrations and the total ion concentration in 0.66 M Mg(NO3)2?
What are the individual ion concentrations and the total ion concentration in 1.04 M Al2(SO4)3?
If the C2H3O2– ion concentration in a solution is 0.554 M, what is the concentration of Ca(C2H3O2)2?
If the Cl− ion concentration in a solution is 2.61 M, what is the concentration of FeCl3?
Molarity is moles per liter, whereas molality is moles per kilogram of solvent.
0.457 M
0.0844 mol
1.59 L
0.376 L
Mg2+ = 0.66 M; NO3− = 1.32 M; total: 1.98 M
0.277 M
Often, a worker will need to change the concentration of a solution by changing the amount of solvent. DilutionThe addition of solvent, which decreases the concentration of the solute in the solution. is the addition of solvent, which decreases the concentration of the solute in the solution. ConcentrationThe removal of solvent, which increases the concentration of the solute in the solution. is the removal of solvent, which increases the concentration of the solute in the solution. (Do not confuse the two uses of the word concentration here!)
In both dilution and concentration, the amount of solute stays the same. This gives us a way to calculate what the new solution volume must be for the desired concentration of solute. From the definition of molarity,
we can solve for the number of moles of solute:
moles of solute = (molarity)(liters of solution)A simpler way of writing this is to use M to represent molarity and V to represent volume. So the equation becomes
moles of solute = MVBecause this quantity does not change before and after the change in concentration, the product MV must be the same before and after the concentration change. Using numbers to represent the initial and final conditions, we have
M1V1 = M2V2as the dilution equationThe mathematical formula for calculating new concentrations or volumes when a solution is diluted or concentrated.. The volumes must be expressed in the same units. Note that this equation gives only the initial and final conditions, not the amount of the change. The amount of change is determined by subtraction.
If 25.0 mL of a 2.19 M solution are diluted to 72.8 mL, what is the final concentration?
Solution
It does not matter which set of conditions is labeled 1 or 2, as long as the conditions are paired together properly. Using the dilution equation, we have
(2.19 M)(25.0 mL) = M2(72.8 mL)Solving for the second concentration (noting that the milliliter units cancel),
M2 = 0.752 MThe concentration of the solution has decreased. In going from 25.0 mL to 72.8 mL, 72.8 − 25.0 = 47.8 mL of solvent must be added.
Test Yourself
A 0.885 M solution of KBr whose initial volume is 76.5 mL has more water added until its concentration is 0.500 M. What is the new volume of the solution?
Answer
135.4 mL
Concentrating solutions involves removing solvent. Usually this is done by evaporating or boiling, assuming that the heat of boiling does not affect the solute. The dilution equation is used in these circumstances as well.
In a hospital emergency room, a physician orders an intravenous (IV) delivery of 100 mL of 0.5% KCl for a patient suffering from hypokalemia (low potassium levels). Does an aide run to a supply cabinet and take out an IV bag containing this concentration of KCl?
Not likely. It is more probable that the aide must make the proper solution from an IV bag of sterile solution and a more concentrated, sterile solution, called a stock solution, of KCl. The aide is expected to use a syringe to draw up some stock solution and inject it into the waiting IV bag and dilute it to the proper concentration. Thus the aide must perform a dilution calculation.
If the stock solution is 10.0% KCl and the final volume and concentration need to be 100 mL and 0.50%, respectively, then it is an easy calculation to determine how much stock solution to use:
(10%)V1 = (0.50%)(100 mL) V1 = 5 mLOf course, the addition of the stock solution affects the total volume of the diluted solution, but the final concentration is likely close enough even for medical purposes.
Medical and pharmaceutical personnel are constantly dealing with dosages that require concentration measurements and dilutions. It is an important responsibility: calculating the wrong dose can be useless, harmful, or even fatal!
What is the difference between dilution and concentration?
What quantity remains constant when you dilute a solution?
A 1.88 M solution of NaCl has an initial volume of 34.5 mL. What is the final concentration of the solution if it is diluted to 134 mL?
A 0.664 M solution of NaCl has an initial volume of 2.55 L. What is the final concentration of the solution if it is diluted to 3.88 L?
If 1.00 mL of a 2.25 M H2SO4 solution needs to be diluted to 1.00 M, what will be its final volume?
If 12.00 L of a 6.00 M HNO3 solution needs to be diluted to 0.750 M, what will be its final volume?
If 665 mL of a 0.875 M KBr solution are boiled gently to concentrate the solute to 1.45 M, what will be its final volume?
If 1.00 L of an LiOH solution is boiled down to 164 mL and its initial concentration is 0.00555 M, what is its final concentration?
How much water must be added to 75.0 mL of 0.332 M FeCl3(aq) to reduce its concentration to 0.250 M?
How much water must be added to 1.55 L of 1.65 M Sc(NO3)3(aq) to reduce its concentration to 1.00 M?
Dilution is a decrease in a solution’s concentration, whereas concentration is an increase in a solution’s concentration.
0.484 M
2.25 mL
401 mL
24.6 mL
Concentration can be a conversion factor between the amount of solute and the amount of solution or solvent (depending on the definition of the concentration unit). As such, concentrations can be useful in a variety of stoichiometry problems. In many cases, it is best to use the original definition of the concentration unit; it is that definition that provides the conversion factor.
A simple example of using a concentration unit as a conversion factor is one in which we use the definition of the concentration unit and rearrange; we can do the calculation again as a unit conversion, rather than as a definition. For example, suppose we ask how many moles of solute are present in 0.108 L of a 0.887 M NaCl solution. Because 0.887 M means 0.887 mol/L, we can use this second expression for the concentration as a conversion factor:
(There is an understood 1 in the denominator of the conversion factor.) If we used the definition approach, we get the same answer, but now we are using conversion factor skills. Like any other conversion factor that relates two different types of units, the reciprocal of the concentration can be also used as a conversion factor.
Using concentration as a conversion factor, how many liters of 2.35 M CuSO4 are needed to obtain 4.88 mol of CuSO4?
Solution
This is a one-step conversion, but the concentration must be written as the reciprocal for the units to work out:
Test Yourself
Using concentration as a conversion factor, how many liters of 0.0444 M CH2O are needed to obtain 0.0773 mol of CH2O?
Answer
1.74 L
Of course, once quantities in moles are available, another conversion can give the mass of the substance, using molar mass as a conversion factor.
What mass of solute is present in 0.765 L of 1.93 M NaOH?
Solution
This is a two-step conversion, first using concentration as a conversion factor to determine the number of moles and then the molar mass of NaOH (40.0 g/mol) to convert to mass:
Test Yourself
What mass of solute is present in 1.08 L of 0.0578 M H2SO4?
Answer
6.12 g
More complex stoichiometry problems using balanced chemical reactions can also use concentrations as conversion factors. For example, suppose the following equation represents a chemical reaction:
2AgNO3(aq) + CaCl2(aq) → 2AgCl(s) + Ca(NO3)2(aq)If we wanted to know what volume of 0.555 M CaCl2 would react with 1.25 mol of AgNO3, we first use the balanced chemical equation to determine the number of moles of CaCl2 that would react and then use concentration to convert to liters of solution:
This can be extended by starting with the mass of one reactant, instead of moles of a reactant.
What volume of 0.0995 M Al(NO3)3 will react with 3.66 g of Ag according to the following chemical equation?
3Ag(s) + Al(NO3)3(aq) → 3AgNO3 + Al(s)Solution
Here, we first must convert the mass of Ag to moles before using the balanced chemical equation and then the definition of molarity as a conversion factor:
The strikeouts show how the units cancel.
Test Yourself
What volume of 0.512 M NaOH will react with 17.9 g of H2C2O4(s) according to the following chemical equation?
H2C2O4(s) + 2NaOH(aq) → Na2C2O4(aq) + 2H2O(ℓ)Answer
0.777 L
We can extend our skills even further by recognizing that we can relate quantities of one solution to quantities of another solution. Knowing the volume and concentration of a solution containing one reactant, we can determine how much of another solution of another reactant will be needed using the balanced chemical equation.
A student takes a precisely measured sample, called an aliquot, of 10.00 mL of a solution of FeCl3. The student carefully adds 0.1074 M Na2C2O4 until all the Fe3+(aq) has precipitated as Fe2(C2O4)3(s). Using a precisely measured tube called a burette, the student finds that 9.04 mL of the Na2C2O4 solution was added to completely precipitate the Fe3+(aq). What was the concentration of the FeCl3 in the original solution? (A precisely measured experiment like this, which is meant to determine the amount of a substance in a sample, is called a titration.) The balanced chemical equation is as follows:
2FeCl3(aq) + 3Na2C2O4(aq) → Fe2(C2O4)3(s) + 6NaCl(aq)Solution
First we need to determine the number of moles of Na2C2O4 that reacted. We will convert the volume to liters and then use the concentration of the solution as a conversion factor:
Now we will use the balanced chemical equation to determine the number of moles of Fe3+(aq) that were present in the initial aliquot:
Then we determine the concentration of FeCl3 in the original solution. Converting 10.00 mL into liters (0.01000 L), we use the definition of molarity directly:
Test Yourself
A student titrates 25.00 mL of H3PO4 with 0.0987 M KOH. She uses 54.06 mL to complete the chemical reaction. What is the concentration of H3PO4?
H3PO4(aq) + 3KOH(aq) → K3PO4(aq) + 3H2OAnswer
0.0711 M
We have used molarity exclusively as the concentration of interest, but that will not always be the case. The next example shows a different concentration unit being used.
H2O2 is used to determine the amount of Mn according to this balanced chemical equation:
2MnO4−(aq) + 5H2O2(aq) + 6H+(aq) → 2Mn2+(aq) + 5O2(g) + 8H2O(ℓ)What mass of 3.00% m/m H2O2 solution is needed to react with 0.355 mol of MnO4−(aq)?
Solution
Because we are given an initial amount in moles, all we need to do is use the balanced chemical equation to determine the number of moles of H2O2 and then convert to find the mass of H2O2. Knowing that the H2O2 solution is 3.00% by mass, we can determine the mass of solution needed:
The first conversion factor comes from the balanced chemical equation, the second conversion factor is the molar mass of H2O2, and the third conversion factor comes from the definition of percentage concentration by mass.
Test Yourself
Use the balanced chemical reaction for MnO4− and H2O2 to determine what mass of O2 is produced if 258 g of 3.00% m/m H2O2 is reacted with MnO4−.
Answer
7.28 g
Using concentration as a conversion factor, how many moles of solute are in 3.44 L of 0.753 M CaCl2?
Using concentration as a conversion factor, how many moles of solute are in 844 mL of 2.09 M MgSO4?
Using concentration as a conversion factor, how many liters are needed to provide 0.822 mol of NaBr from a 0.665 M solution?
Using concentration as a conversion factor, how many liters are needed to provide 2.500 mol of (NH2)2CO from a 1.087 M solution?
What is the mass of solute in 24.5 mL of 0.755 M CoCl2?
What is the mass of solute in 3.81 L of 0.0232 M Zn(NO3)2?
What volume of solution is needed to provide 9.04 g of NiF2 from a 0.332 M solution?
What volume of solution is needed to provide 0.229 g of CH2O from a 0.00560 M solution?
What volume of 3.44 M HCl will react with 5.33 mol of CaCO3?
2HCl + CaCO3 → CaCl2 + H2O + CO2What volume of 0.779 M NaCl will react with 40.8 mol of Pb(NO3)2?
Pb(NO3)2 + 2NaCl → PbCl2 + 2NaNO3What volume of 0.905 M H2SO4 will react with 26.7 mL of 0.554 M NaOH?
H2SO4 + 2NaOH → Na2SO4 + 2H2OWhat volume of 1.000 M Na2CO3 will react with 342 mL of 0.733 M H3PO4?
3Na2CO3 + 2H3PO4 → 2Na3PO4 + 3H2O + 3CO2It takes 23.77 mL of 0.1505 M HCl to titrate with 15.00 mL of Ca(OH)2. What is the concentration of Ca(OH)2? You will need to write the balanced chemical equation first.
It takes 97.62 mL of 0.0546 M NaOH to titrate a 25.00 mL sample of H2SO4. What is the concentration of H2SO4? You will need to write the balanced chemical equation first.
It takes 4.667 mL of 0.0997 M HNO3 to dissolve some solid Cu. What mass of Cu can be dissolved?
Cu + 4HNO3(aq) → Cu(NO3)2(aq) + 2NO2 + 2H2OIt takes 49.08 mL of 0.877 M NH3 to dissolve some solid AgCl. What mass of AgCl can be dissolved?
AgCl(s) + 4NH3(aq) → Ag(NH3)4Cl(aq)What mass of 3.00% H2O2 is needed to produce 66.3 g of O2(g)?
2H2O2(aq) → 2H2O(ℓ) + O2(g)A 0.75% solution of Na2CO3 is used to precipitate Ca2+ ions from solution. What mass of solution is needed to precipitate 40.7 L of solution with a concentration of 0.0225 M Ca2+(aq)?
Na2CO3(aq) + Ca2+(aq) → CaCO3(s) + 2Na+(aq)2.59 mol
1.24 L
2.40 g
0.282 L
3.10 L
8.17 mL
0.1192 M
7.39 mg
4.70 kg
The properties of solutions are very similar to the properties of their respective pure solvents. This makes sense because the majority of the solution is the solvent. However, some of the properties of solutions differ from pure solvents in measurable and predictable ways. The differences are proportional to the fraction that the solute particles occupy in the solution. These properties are called colligative propertiesA property of solutions related to the fraction that the solute particles occupy in the solution, not their identity.; the word colligative comes from the Greek word meaning “related to the number,” implying that these properties are related to the number of solute particles, not their identities.
Before we introduce the first colligative property, we need to introduce a new concentration unit. The mole fractionThe ratio of the number of moles of a component to the total number of moles in a system. of the ith component in a solution, χi, is the number of moles of that component divided by the total number of moles in the sample:
(χ is the lowercase Greek letter chi.) The mole fraction is always a number between 0 and 1 (inclusive) and has no units; it is just a number.
A solution is made by mixing 12.0 g of C10H8 in 45.0 g of C6H6. What is the mole fraction of C10H8 in the solution?
Solution
We need to determine the number of moles of each substance, add them together to get the total number of moles, and then divide to determine the mole fraction of C10H8. The number of moles of C10H8 is as follows:
The number of moles of C6H6 is as follows:
The total number of moles is
0.0936 mol + 0.576 mol = 0.670 molNow we can calculate the mole fraction of C10H8:
The mole fraction is a number between 0 and 1 and is unitless.
Test Yourself
A solution is made by mixing 33.8 g of CH3OH in 50.0 g of H2O. What is the mole fraction of CH3OH in the solution?
Answer
0.275
A useful thing to note is that the sum of the mole fractions of all substances in a mixture equals 1. Thus the mole fraction of C6H6 in Example 15 could be calculated by evaluating the definition of mole fraction a second time, or—because there are only two substances in this particular mixture—we can subtract the mole fraction of the C10H8 from 1 to get the mole fraction of C6H6.
Now that this new concentration unit has been introduced, the first colligative property can be considered. As was mentioned in Chapter 10 "Solids and Liquids", all pure liquids have a characteristic vapor pressure in equilibrium with the liquid phase, the partial pressure of which is dependent on temperature. Solutions, however, have a lower vapor pressure than the pure solvent has, and the amount of lowering is dependent on the fraction of solute particles, as long as the solute itself does not have a significant vapor pressure (the term nonvolatile is used to describe such solutes). This colligative property is called vapor pressure depressionThe decrease of a solution’s vapor pressure because of the presence of a solute. (or lowering). The actual vapor pressure of the solution can be calculated as follows:
where Psoln is the vapor pressure of the solution, χsolv is the mole fraction of the solvent particles, and is the vapor pressure of the pure solvent at that temperature (which is data that must be provided). This equation is known as Raoult’s lawThe mathematical formula for calculating the vapor pressure of a solution. (the approximate pronunciation is rah-OOLT). Vapor pressure depression is rationalized by presuming that solute particles take positions at the surface in place of solvent particles, so not as many solvent particles can evaporate.
A solution is made by mixing 12.0 g of C10H8 in 45.0 g of C6H6. If the vapor pressure of pure C6H6 is 95.3 torr, what is the vapor pressure of the solution?
Solution
This is the same solution that was in Example 15, but here we need the mole fraction of C6H6. The number of moles of C10H8 is as follows:
The number of moles of C6H6 is as follows:
So the total number of moles is
0.0936 mol + 0.576 mol = 0.670 molNow we can calculate the mole fraction of C6H6:
(The mole fraction of C10H8 calculated in Example 15 plus the mole fraction of C6H6 equals 1, which is mathematically required by the definition of mole fraction.) Now we can use Raoult’s law to determine the vapor pressure in equilibrium with the solution:
Psoln = (0.860)(95.3 torr) = 82.0 torrThe solution has a lower vapor pressure than the pure solvent.
Test Yourself
A solution is made by mixing 33.8 g of C6H12O6 in 50.0 g of H2O. If the vapor pressure of pure water is 25.7 torr, what is the vapor pressure of the solution?
Answer
24.1 torr
Two colligative properties are related to solution concentration as expressed in molality. As a review, recall the definition of molality:
Because the vapor pressure of a solution with a nonvolatile solute is depressed compared to that of the pure solvent, it requires a higher temperature for the solution’s vapor pressure to reach 1.00 atm (760 torr). Recall that this is the definition of the normal boiling point: the temperature at which the vapor pressure of the liquid equals 1.00 atm. As such, the normal boiling point of the solution is higher than that of the pure solvent. This property is called boiling point elevationThe increase of a solution’s boiling point because of the presence of solute..
The change in boiling point (ΔTb) is easily calculated:
ΔTb = mKbwhere m is the molality of the solution and Kb is called the boiling point elevation constantThe constant that relates the molality concentration of a solution and its boiling point change., which is a characteristic of the solvent. Several boiling point elevation constants (as well as boiling point temperatures) are listed in Table 11.3 "Boiling Point Data for Various Liquids".
Table 11.3 Boiling Point Data for Various Liquids
| Liquid | Boiling Point (°C) | Kb (°C/m) |
|---|---|---|
| HC2H3O2 | 117.90 | 3.07 |
| C6H6 | 80.10 | 2.53 |
| CCl4 | 76.8 | 4.95 |
| H2O | 100.00 | 0.512 |
Remember that what is initially calculated is the change in boiling point temperature, not the new boiling point temperature. Once the change in boiling point temperature is calculated, it must be added to the boiling point of the pure solvent—because boiling points are always elevated—to get the boiling point of the solution.
What is the boiling point of a 2.50 m solution of C6H4Cl2 in CCl4? Assume that C6H4Cl2 is not volatile.
Solution
Using the equation for the boiling point elevation,
ΔTb = (2.50 m)(4.95°C/m) = 12.4°CNote how the molality units have canceled. However, we are not finished. We have calculated the change in the boiling point temperature, not the final boiling point temperature. If the boiling point goes up by 12.4°C, we need to add this to the normal boiling point of CCl4 to get the new boiling point of the solution:
TBP = 76.8°C + 12.4°C = 89.2°CThe boiling point of the solution is predicted to be 89.2°C.
Test Yourself
What is the boiling point of a 6.95 m solution of C12H22O11 in H2O?
Answer
103.6°C
The boiling point of a solution is higher than the boiling point of the pure solvent, but the opposite occurs with the freezing point. The freezing point of a solution is lower than the freezing point of the pure solvent. Think of this by assuming that solute particles interfere with solvent particles coming together to make a solid, so it takes a lower temperature to get the solvent particles to solidify. This is called freezing point depressionThe decrease of a solution’s freezing point because of the presence of solute..
The equation to calculate the change in the freezing point for a solution is similar to the equation for the boiling point elevation:
ΔTf = mKfwhere m is the molality of the solution and Kf is called the freezing point depression constantThe constant that relates the molality concentration of a solution and its freezing point change., which is also a characteristic of the solvent. Several freezing point depression constants (as well as freezing point temperatures) are listed in Table 11.4 "Freezing Point Data for Various Liquids".
Table 11.4 Freezing Point Data for Various Liquids
| Liquid | Freezing Point (°C) | Kf (°C/m) |
|---|---|---|
| HC2H3O2 | 16.60 | 3.90 |
| C6H6 | 5.51 | 4.90 |
| C6H12 | 6.4 | 20.2 |
| C10H8 | 80.2 | 6.8 |
| H2O | 0.00 | 1.86 |
Remember that this equation calculates the change in the freezing point, not the new freezing point. What is calculated needs to be subtracted from the normal freezing point of the solvent because freezing points always go down.
What is the freezing point of a 1.77 m solution of CBr4 in C6H6?
Solution
We use the equation to calculate the change in the freezing point and then subtract this number from the normal freezing point of C6H6 to get the freezing point of the solution:
ΔTf = (1.77 m)(4.90°C/m) = 8.67°CNow we subtract this number from the normal freezing point of C6H6, which is 5.51°C:
5.51 − 8.67 = −3.16°CThe freezing point of the solution is −3.16°C.
Test Yourself
What is the freezing point of a 3.05 m solution of CBr4 in C10H8?
Answer
59.5°C
Freezing point depression is one colligative property we use in everyday life. Many antifreezes used in automobile radiators use solutions that have a lower freezing point than normal so that automobile engines can operate at subfreezing temperatures. We also take advantage of freezing point depression when we sprinkle various compounds on ice to thaw it in the winter for safety (Figure 11.2 "Salt and Safety"). The compounds make solutions that have a lower freezing point, so rather than forming slippery ice, any ice is liquefied and runs off, leaving a safer pavement behind.
Before we introduce the final colligative property, we need to present a new concept. A semipermeable membraneA thin membrane that will pass certain small molecules but not others. is a thin membrane that will pass certain small molecules but not others. A thin sheet of cellophane, for example, acts as a semipermeable membrane.
Consider the system in Figure 11.3 "Osmosis"a. A semipermeable membrane separates two solutions having the different concentrations marked. Curiously, this situation is not stable; there is a tendency for water molecules to move from the dilute side (on the left) to the concentrated side (on the right) until the concentrations are equalized, as in Figure 11.3 "Osmosis"b. This tendency is called osmosisThe tendency of solvent molecules to pass through a semipermeable membrane due to concentration differences.. In osmosis, the solute remains in its original side of the system; only solvent molecules move through the semipermeable membrane. In the end, the two sides of the system will have different volumes. Because a column of liquid exerts a pressure, there is a pressure difference Π on the two sides of the system that is proportional to the height of the taller column. This pressure difference is called the osmotic pressureThe tendency of a solution to pass solvent through a semipermeable membrane due to concentration differences., which is a colligative property.
Figure 11.3 Osmosis
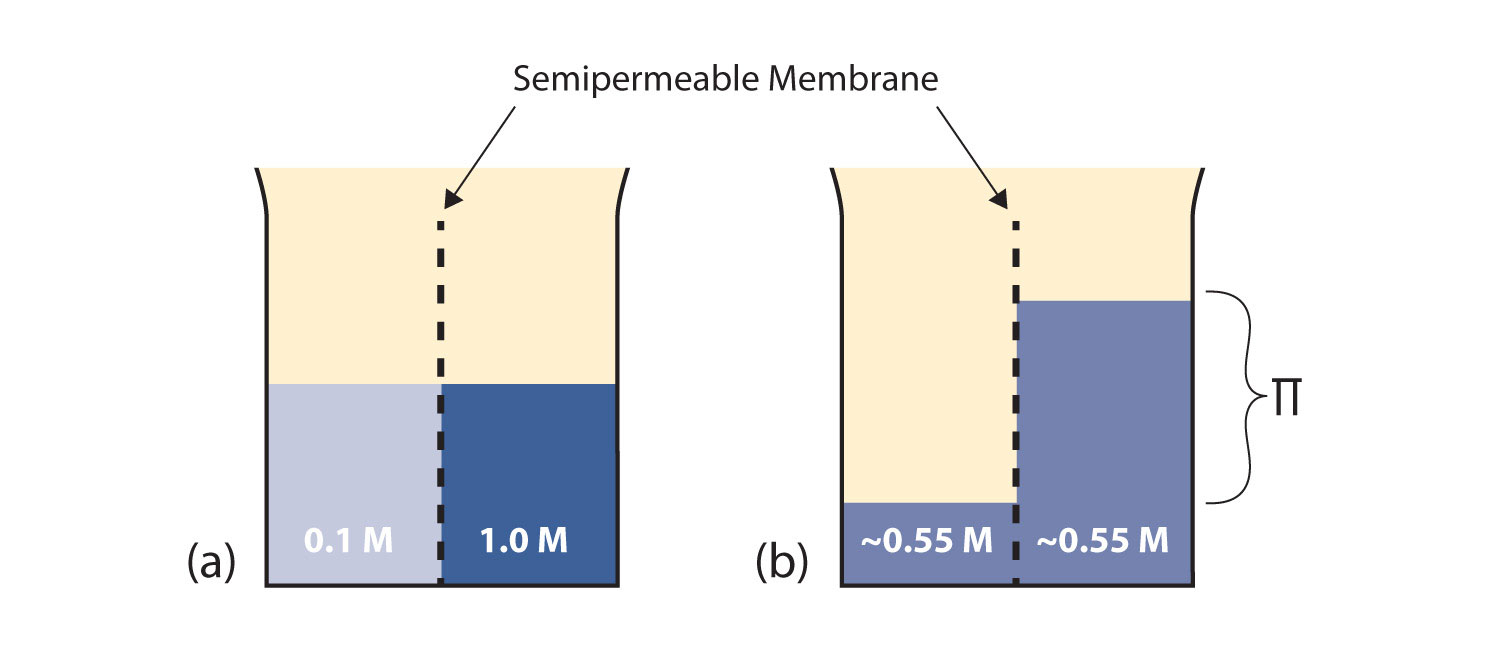
(a) Two solutions of differing concentrations are placed on either side of a semipermeable membrane. (b) When osmosis occurs, solvent molecules selectively pass through the membrane from the dilute solution to the concentrated solution, diluting it until the two concentrations are the same. The pressure exerted by the different height of the solution on the right is called the osmotic pressure.
The osmotic pressure of a solution is easy to calculate:
Π = MRTwhere Π is the osmotic pressure of a solution, M is the molarity of the solution, R is the ideal gas law constant, and T is the absolute temperature. This equation is reminiscent of the ideal gas law we considered in Chapter 6 "Gases".
What is the osmotic pressure of a 0.333 M solution of C6H12O6 at 25°C?
Solution
First we need to convert our temperature to kelvins:
T = 25 + 273 = 298 KNow we can substitute into the equation for osmotic pressure, recalling the value for R:
The units may not make sense until we realize that molarity is defined as moles per liter:
Now we see that the moles, liters, and kelvins cancel, leaving atmospheres, which is a unit of pressure. Solving,
Π = 8.14 atmThis is a substantial pressure! It is the equivalent of a column of water 84 m tall.
Test Yourself
What is the osmotic pressure of a 0.0522 M solution of C12H22O11 at 55°C?
Answer
1.40 atm
Osmotic pressure is important in biological systems because cell walls are semipermeable membranes. In particular, when a person is receiving intravenous (IV) fluids, the osmotic pressure of the fluid needs to be approximately the same as blood serum; otherwise bad things can happen. Figure 11.4 "Osmotic Pressure and Red Blood Cells" shows three red blood cells: Figure 11.4 "Osmotic Pressure and Red Blood Cells"a shows a healthy red blood cell. Figure 11.4 "Osmotic Pressure and Red Blood Cells"b shows a red blood cell that has been exposed to a lower concentration than normal blood serum (a so-called hypotonic solution); the cell has plumped up as solvent moves into the cell to dilute the solutes inside. Figure 11.4 "Osmotic Pressure and Red Blood Cells"c shows a red blood cell exposed to a higher concentration than normal blood serum (hypertonic); water leaves the red blood cell, so it collapses onto itself. Only when the solutions inside and outside the cell are the same (isotonic) will the red blood cell be able to do its job.
Figure 11.4 Osmotic Pressure and Red Blood Cells
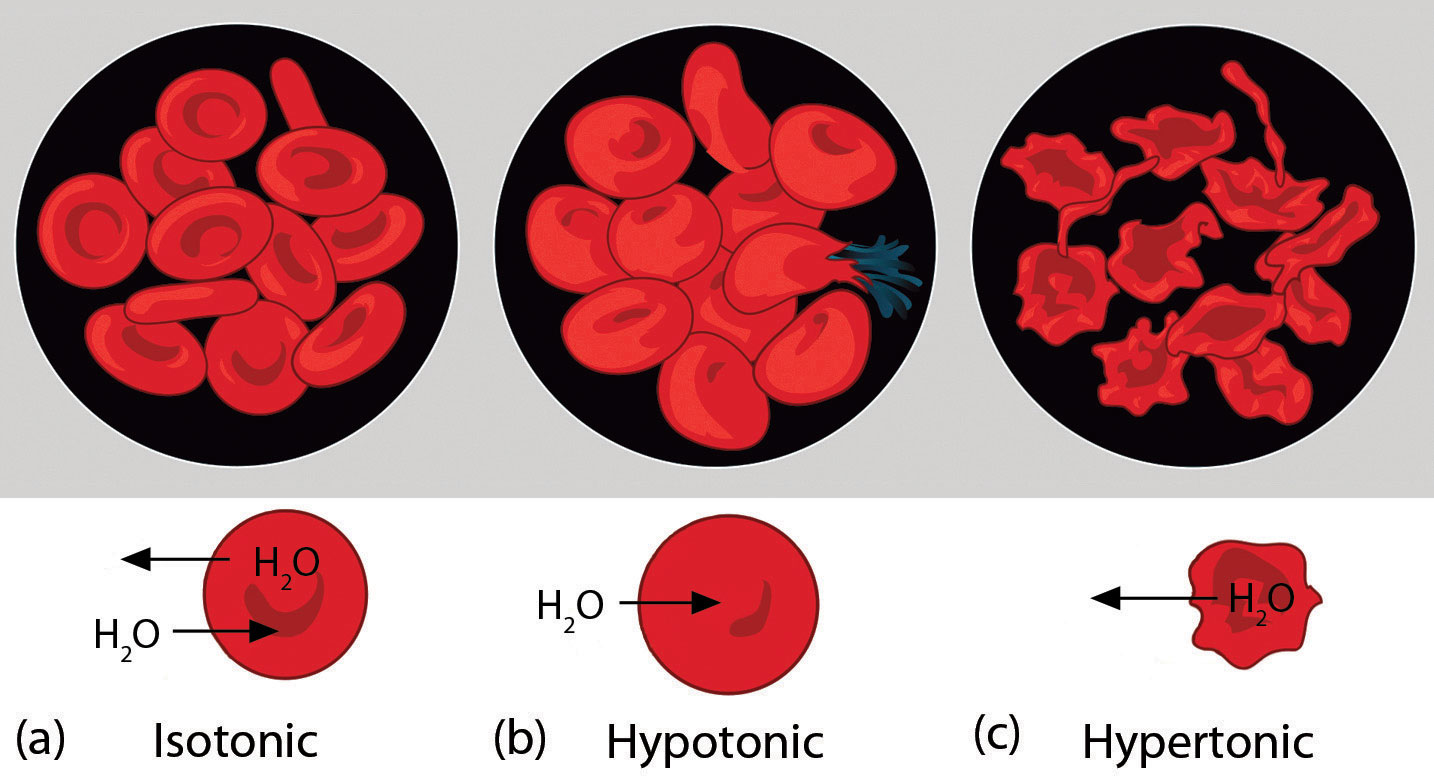
(a) This is what a normal red blood cell looks like. (b) When a red blood cell is exposed to a hypotonic solution, solvent goes through the cell membrane and dilutes the inside of the cell. (c) When a red blood cell is exposed to a hypertonic solution, solvent goes from the cell to the surrounding solution, diluting the hypertonic solution and collapsing the cell. Neither of these last two cases is desirable, so IV solutions must be isotonic with blood serum to not cause deleterious effects.
Osmotic pressure is also the reason you should not drink seawater if you’re stranded in a lifeboat on an ocean; seawater has a higher osmotic pressure than most of the fluids in your body. You can drink the water, but ingesting it will pull water out of your cells as osmosis works to dilute the seawater. Ironically, your cells will die of thirst, and you will also die. (It is OK to drink the water if you are stranded on a body of freshwater, at least from an osmotic pressure perspective.) Osmotic pressure is also thought to be important—in addition to capillary action—in getting water to the tops of tall trees.
What are the three colligative properties that involve phase changes?
Which colligative property does not involve a phase change? Give an example of its importance.
If 45.0 g of C6H6 and 60.0 g of C6H5CH3 are mixed together, what is the mole fraction of each component?
If 125 g of N2 are mixed with 175 g of O2, what is the mole fraction of each component?
If 36.5 g of NaCl are mixed with 63.5 g of H2O, what is the mole fraction of each component?
An alloy of stainless steel is prepared from 75.4 g of Fe, 12.6 g of Cr, and 10.8 g of C. What is the mole fraction of each component?
A solution is made by mixing 12.0 g of C10H8 in 45.0 g of C6H6. If the vapor pressure of pure C6H6 is 76.5 torr at a particular temperature, what is the vapor pressure of the solution at the same temperature?
A solution is made by mixing 43.9 g of C6H12C6 in 100.0 g of H2O. If the vapor pressure of pure water is 26.5 torr at a particular temperature, what is the vapor pressure of the solution at the same temperature?
At 300°C, the vapor pressure of Hg is 32.97 torr. If 0.775 g of Au were dissolved into 3.77 g of Hg, what would be the vapor pressure of the solution?
At 300°C, the vapor pressure of Hg is 32.97 torr. What mass of Au would have to be dissolved in 5.00 g of Hg to lower its vapor pressure to 25.00 torr?
If 25.0 g of C6H12O6 are dissolved in 100.0 g of H2O, what is the boiling point of this solution?
If 123 g of C10H16O are dissolved in 355 g of C6H6, what is the boiling point of this solution?
If 1 mol of solid CBr4 is mixed with 2 mol of CCl4, what is the boiling point of this solution?
A solution of C2H2O4 in CH3COOH has a boiling point of 123.40°C. What is the molality of the solution?
If 123 g of C10H16O are dissolved in 355 g of C6H6, what is the freezing point of this solution?
If 25.0 g of C6H12O6 are dissolved in 100.0 g of H2O, what is the freezing point of this solution?
C8H17OH is a nonvolatile solid that dissolves in C6H12. If 7.22 g of C8H17OH is dissolved in 45.3 g of C6H12, what is the freezing point of this solution?
A solution of C2H2O4 in CH3COOH has a freezing point of 10.00°C. What is the molality of the solution?
If 25.0 g of C6H12O6 are dissolved in H2O to make 0.100 L of solution, what is the osmotic pressure of this solution at 25°C?
If 2.33 g of C27H46O are dissolved in liquid CS2 to make 50.00 mL of solution, what is the osmotic pressure of this solution at 298 K?
At 298 K, what concentration of solution is needed to have an osmotic pressure of 1.00 atm?
The osmotic pressure of blood is about 7.65 atm at 37°C. What is the approximate concentration of dissolved solutes in blood? (There are many different solutes in blood, so the answer is indeed an approximation.)
boiling point elevation, freezing point depression, vapor pressure depression
mole fraction C6H6: 0.469; mole fraction C6H5CH3: 0.531
mole fraction NaCl: 0.157; mole fraction H2O: 0.843
65.8 torr
27.26 torr
100.71°C
92.9°C
−5.65°C
−18.3°C
33.9 atm
0.0409 M
In Section 11.5 "Colligative Properties of Solutions", we considered the colligative properties of solutions with molecular solutes. What about solutions with ionic solutes? Do they exhibit colligative properties?
There is a complicating factor: ionic solutes separate into ions when they dissolve. This increases the total number of particles dissolved in solution and increases the impact on the resulting colligative property. Historically, this greater-than-expected impact on colligative properties was one main piece of evidence for ionic compounds separating into ions (increased electrical conductivity was another piece of evidence).
For example, when NaCl dissolves, it separates into two ions:
NaCl(s) → Na+(aq) + Cl−(aq)This means that a 1 M solution of NaCl actually has a net particle concentration of 2 M. The observed colligative property will then be twice as large as expected for a 1 M solution.
It is easy to incorporate this concept into our equations to calculate the respective colligative property. We define the van’t Hoff factorThe number of particles each solute formula unit breaks apart into when it dissolves. (i) as the number of particles each solute formula unit breaks apart into when it dissolves. Previously, we have always tacitly assumed that the van’t Hoff factor is simply 1. But for some ionic compounds, i is not 1, as shown in Table 11.5 "Ideal van’t Hoff Factors for Ionic Compounds".
Table 11.5 Ideal van’t Hoff Factors for Ionic Compounds
| Compound | i |
|---|---|
| NaCl | 2 |
| KBr | 2 |
| LiNO3 | 2 |
| CaCl2 | 3 |
| Mg(C2H3O2)2 | 3 |
| FeCl3 | 4 |
| Al2(SO4)3 | 5 |
The ideal van’t Hoff factor is equal to the number of ions that form when an ionic compound dissolves.
Predict the van’t Hoff factor for Sr(OH)2.
Solution
When Sr(OH)2 dissolves, it separates into one Sr2+ ion and two OH− ions:
Sr(OH)2 → Sr2+(aq) + 2OH−(aq)Because it breaks up into three ions, its van’t Hoff factor is 3.
Test Yourself
What is the van’t Hoff factor for Fe(NO3)3?
Answer
4
It is the “ideal” van’t Hoff factor because this is what we expect from the ionic formula. However, this factor is usually correct only for dilute solutions (solutions less than 0.001 M). At concentrations greater than 0.001 M, there are enough interactions between ions of opposite charge that the net concentration of the ions is less than expected—sometimes significantly. The actual van’t Hoff factor is thus less than the ideal one. Here, we will use ideal van’t Hoff factors.
Revised equations to calculate the effect of ionization are then easily produced:
ΔTb = imKb ΔTf = imKg Π = iMRTwhere all variables have been previously defined. To calculate vapor pressure depression according to Raoult’s law, the mole fraction of solvent particles must be recalculated to take into account the increased number of particles formed on ionization.
Determine the freezing point of a 1.77 m solution of NaCl in H2O.
Solution
For NaCl, we need to remember to include the van’t Hoff factor, which is 2. Otherwise, the calculation of the freezing point is straightforward:
ΔTf = (2)(1.77 m)(1.86°C/m) = 6.58°CThis represents the change in the freezing point, which is decreasing. So we have to subtract this change from the normal freezing point of water, 0.00°C:
0.00 − 6.58 = −6.58°CTest Yourself
Determine the boiling point of a 0.887 m solution of CaCl2 in H2O.
Answer
101.36°C
When cooking dried pasta, many recipes call for salting the water before cooking the pasta. Some argue—with colligative properties on their side—that adding salt to the water raises the boiling point, thus cooking the pasta faster. Is there any truth to this?
To judge the veracity of this claim, we can calculate how much salt should be added to the water to raise the boiling temperature by 1.0°C, with the presumption that dried pasta cooks noticeably faster at 101°C than at 100°C (although a 1° difference may make only a negligible change in cooking times). We can calculate the molality that the water should have:
1.0°C = m(0.512°C/m) m = 1.95We have ignored the van’t Hoff factor in our estimation because this obviously is not a dilute solution. Let us further assume that we are using 4 L of water (which is very close to 4 qt, which in turn equals 1 gal). Because 4 L of water is about 4 kg (it is actually slightly less at 100°C), we can determine how much salt (NaCl) to add:
This is just over 1 lb of salt and is equivalent to nearly 1 cup in the kitchen. In your experience, do you add almost a cup of salt to a pot of water to make pasta? Certainly not! A few pinches, perhaps one-fourth of a teaspoon, but not almost a cup! It is obvious that the little amount of salt that most people add to their pasta water is not going to significantly raise the boiling point of the water.
So why do people add some salt to boiling water? There are several possible reasons, the most obvious of which is taste: adding salt adds a little bit of salt flavor to the pasta. It cannot be much because most of the salt remains in the water, not in the cooked pasta. However, it may be enough to detect with our taste buds. The other obvious reason is habit; recipes tell us to add salt, so we do, even if there is little scientific or culinary reason to do so.
Explain why we need to consider a van’t Hoff factor for ionic solutes but not for molecular solutes.
NaCl is often used in winter to melt ice on roads and sidewalks, but calcium chloride (CaCl2) is also used. Which would be better (on a mole-by-mole basis), and why?
Calculate the boiling point of an aqueous solution of NaNO3 made by mixing 15.6 g of NaNO3 with 100.0 g of H2O. Assume an ideal van’t Hoff factor.
Many labs use a cleaning solution of KOH dissolved in C2H5OH. If 34.7 g of KOH were dissolved in 88.0 g of C2H5OH, what is the boiling point of this solution? The normal boiling point of C2H5OH is 78.4°C and its Kb = 1.19°C/m. Assume an ideal van’t Hoff factor.
What is the freezing point of a solution made by dissolving 345 g of CaCl2 in 1,550 g of H2O? Assume an ideal van’t Hoff factor.
A classic homemade ice cream can be made by freezing the ice cream mixture using a solution of 250 g of NaCl dissolved in 1.25 kg of ice water. What is the temperature of this ice water? Assume an ideal van’t Hoff factor.
Seawater can be approximated as a 3.5% NaCl solution by mass; that is, 3.5 g of NaCl are combined with 96.5 g H2O. What is the osmotic pressure of seawater? Assume an ideal van’t Hoff factor.
The osmotic pressure of blood is 7.65 atm at 37°C. If blood were considered a solution of NaCl, what is the molar concentration of NaCl in blood? Assume an ideal van’t Hoff factor.
What is the vapor pressure of an aqueous solution of 36.4 g of KBr in 199.5 g of H2O if the vapor pressure of H2O at the same temperature is 32.55 torr? What other solute(s) would give a solution with the same vapor pressure? Assume an ideal van’t Hoff factor.
Assuming an ideal van’t Hoff factor, what mole fraction is required for a solution of Mg(NO3)2 to have a vapor pressure of 20.00 torr at 25.0°C? The vapor pressure of the solvent is 23.61 torr at this temperature.
Ionic solutes separate into more than one particle when they dissolve, whereas molecular solutes do not.
101.9°C
−7.5°C
30.3 atm
30.86 torr; any two-ion salt should have the same effect.
One brand of ethyl alcohol (Everclear) is 95% ethyl alcohol, with the remaining 5% being water. What is the solvent and what is the solute of this solution?
Give an example of each type of solution from your own experience.
Differentiate between the terms saturated and concentrated.
Differentiate between the terms unsaturated and dilute.
What mass of FeCl2 is present in 445 mL of 0.0812 M FeCl2 solution?
What mass of SO2 is present in 26.8 L of 1.22 M SO2 solution?
What volume of 0.225 M Ca(OH)2 solution is needed to deliver 100.0 g of Ca(OH)2?
What volume of 12.0 M HCl solution is needed to obtain exactly 1.000 kg of HCl?
The World Health Organization recommends that the maximum fluoride ion concentration in drinking water is 1.0 ppm. Assuming water has the maximum concentration, if an average person drinks 1,920 mL of water per day, how many milligrams of fluoride ion are being ingested?
For sanitary reasons, water in pools should be chlorinated to a maximum level of 3.0 ppm. In a typical 5,000 gal pool that contains 21,200 kg of water, what mass of chlorine must be added to obtain this concentration?
Given its notoriety, you might think that uranium is very rare, but it is present at about 2–4 ppm of the earth’s crust, which is more abundant than silver or mercury. If the earth’s crust is estimated to have a mass of 8.50 × 1020 kg, what range of mass is thought to be uranium in the crust?
Chromium is thought to be an ultratrace element, with about 8.9 ng present in a human body. If the average body mass is 75.0 kg, what is the concentration of chromium in the body in pptr?
What mass of 3.00% H2O2 solution is needed to produce 35.7 g of O2(g) at 295 K at 1.05 atm pressure?
2H2O2(aq) → 2H2O(ℓ) + O2(g)What volume of pool water is needed to generate 1.000 L of Cl2(g) at standard temperature and pressure if the pool contains 4.0 ppm HOCl and the water is slightly acidic? The chemical reaction is as follows:
HOCl(aq) + HCl(aq) → H2O(ℓ) + Cl2(g)Assume the pool water has a density of 1.00 g/mL.
A 0.500 m solution of MgCl2 has a freezing point of −2.60°C. What is the true van’t Hoff factor of this ionic compound? Why is it less than the ideal value?
The osmotic pressure of a 0.050 M LiCl solution at 25.0°C is 2.26 atm. What is the true van’t Hoff factor of this ionic compound? Why is it less than the ideal value?
Order these solutions in order of increasing boiling point, assuming an ideal van’t Hoff factor for each: 0.10 m C6H12O6, 0.06 m NaCl, 0.4 m Au(NO3)3, and 0.4 m Al2(SO4)3.
Order these solutions in order of decreasing osmotic pressure, assuming an ideal van’t Hoff factor: 0.1 M HCl, 0.1 M CaCl2, 0.05 M MgBr2, and 0.07 M Ga(C2H3O2)3
solvent: ethyl alcohol; solute: water
Saturated means all the possible solute that can dissolve is dissolved, whereas concentrated implies that a lot of solute is dissolved.
4.58 g
6.00 L
1.92 mg
1.7 × 1015 to 3.4 × 1015 kg
2,530 g
2.80; it is less than 3 because not all ions behave as independent particles.
0.10 m C6H12O6 < 0.06 m NaCl < 0.4 m Au(NO3)3 < 0.4 m Al2(SO4)3