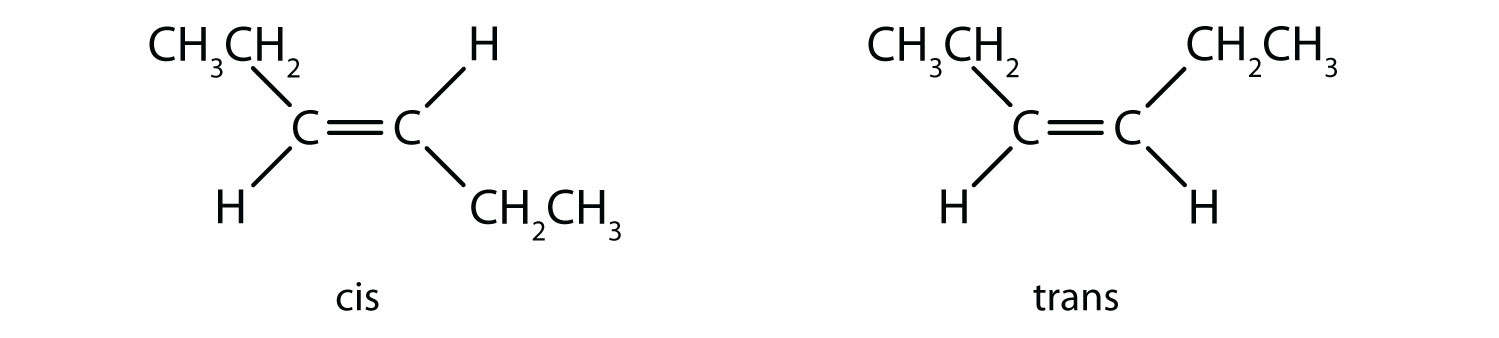As noted in Chapter 12 "Organic Chemistry: Alkanes and Halogenated Hydrocarbons", there is free rotation about the carbon-to-carbon single bonds (C–C) in alkanes. In contrast, the structure of alkenes requires that the carbon atoms of a double bond and the two atoms bonded to each carbon atom all lie in a single plane, and that each doubly bonded carbon atom lies in the center of a triangle. This part of the molecule’s structure is rigid; rotation about doubly bonded carbon atoms is not possible without rupturing the bond. Look at the two chlorinated hydrocarbons in Figure 13.2 "Rotation about Bonds".
Figure 13.2 Rotation about Bonds

In 1,2-dichloroethane (a), free rotation about the C–C bond allows the two structures to be interconverted by a twist of one end relative to the other. In 1,2-dichloroethene (b), restricted rotation about the double bond means that the relative positions of substituent groups above or below the double bond are significant.
In 1,2-dichloroethane (part (a) of Figure 13.2 "Rotation about Bonds"), there is free rotation about the C–C bond. The two models shown represent exactly the same molecule; they are not isomers. You can draw structural formulas that look different, but if you bear in mind the possibility of this free rotation about single bonds, you should recognize that these two structures represent the same molecule:

In 1,2-dichloroethene (part (b) of Figure 13.2 "Rotation about Bonds"), however, restricted rotation about the double bond means that the relative positions of substituent groups above or below the double bond become significant. This leads to a special kind of isomerism. The isomer in which the two chlorine (Cl) atoms lie on the same side of the molecule is called the cis isomerAn isomer in which two substituent groups are attached on the same side of a double bond or ring in an organic molecule. (Latin cis, meaning “on this side”) and is named cis-1,2-dichloroethene. The isomer with the two Cl atoms on opposite sides of the molecule is the trans isomerAn isomer in which two substituent groups are attached to opposite sides of a double bond or ring in a molecule. (Latin trans, meaning “across”) and is named trans-1,2-dichloroethene. These two compounds are cis-trans isomers (or geometric isomers)Isomers that have different configurations because of the presence of a rigid structure such as a double bond or ring., compounds that have different configurations (groups permanently in different places in space) because of the presence of a rigid structure in their molecule.
Consider the alkene with the condensed structural formula CH3CH=CHCH3. We could name it 2-butene, but there are actually two such compounds; the double bond results in cis-trans isomerism (Figure 13.3 "Ball-and-Spring Models of (a) Cis-2-Butene and (b) Trans-2-Butene").
Figure 13.3 Ball-and-Spring Models of (a) Cis-2-Butene and (b) Trans-2-Butene

Cis-trans isomers have different physical, chemical, and physiological properties.
Cis-2-butene has both methyl groups on the same side of the molecule. Trans-2-butene has the methyl groups on opposite sides of the molecule. Their structural formulas are as follows:

Note, however, that the presence of a double bond does not necessarily lead to cis-trans isomerism. We can draw two seemingly different propenes:

However, these two structures are not really different from each other. If you could pick up either molecule from the page and flip it over top to bottom, you would see that the two formulas are identical.
Thus there are two requirements for cis-trans isomerism:
In these propene structures, the second requirement for cis-trans isomerism is not fulfilled. One of the doubly bonded carbon atoms does have two different groups attached, but the rules require that both carbon atoms have two different groups.
In general, the following statements hold true in cis-trans isomerism:
Cis-trans isomerism also occurs in cyclic compounds. In ring structures, groups are unable to rotate about any of the ring carbon–carbon bonds. Therefore, groups can be either on the same side of the ring (cis) or on opposite sides of the ring (trans). For our purposes here, we represent all cycloalkanes as planar structures, and we indicate the positions of the groups, either above or below the plane of the ring.

Which compounds can exist as cis-trans (geometric) isomers? Draw them.
Solution
All four structures have a double bond and thus meet rule 1 for cis-trans isomerism.
This compound meets rule 2; it has two nonidentical groups on each carbon atom (H and Cl on one and H and Br on the other). It exists as both cis and trans isomers:

This compound meets rule 2; it has two nonidentical groups on each carbon atom and exists as both cis and trans isomers:

Which compounds can exist as cis-trans isomers? Draw them.


What are cis-trans (geometric) isomers? What two types of compounds can exhibit cis-trans isomerism?
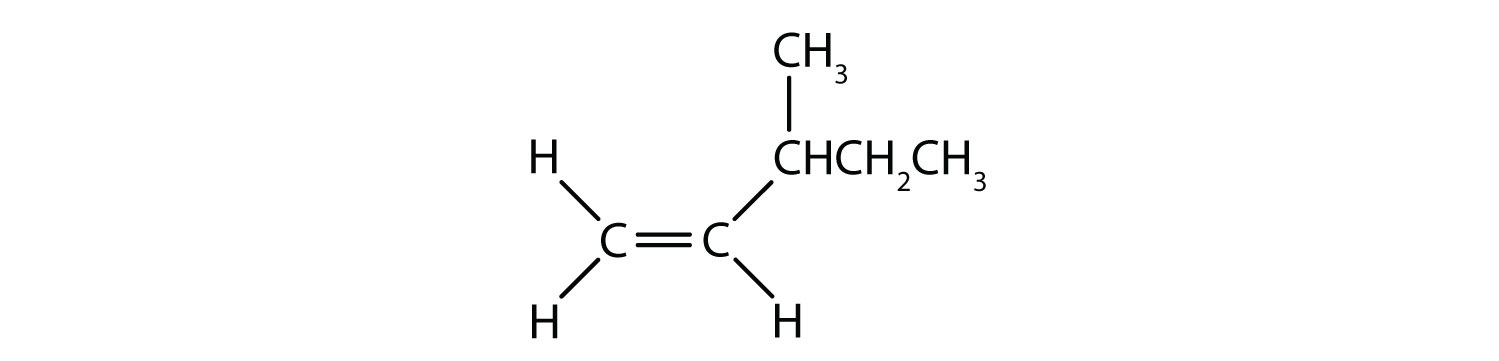
Classify each compound as a cis isomer, a trans isomer, or neither.



Cis-trans isomers are compounds that have different configurations (groups permanently in different places in space) because of the presence of a rigid structure in their molecule. Alkenes and cyclic compounds can exhibit cis-trans isomerism.
Draw the structures of the cis-trans isomers for each compound. Label them cis and trans. If no cis-trans isomers exist, write none.
Draw the structures of the cis-trans isomers for each compound. Label them cis and trans. If no cis-trans isomers exist, write none.