The simplest alkyne—a hydrocarbon with carbon-to-carbon triple bond—has the molecular formula C2H2 and is known by its common name—acetylene (Figure 13.5 "Ball-and-Spring Model of Acetylene"). Its structure is H–C≡C–H.
Figure 13.5 Ball-and-Spring Model of Acetylene
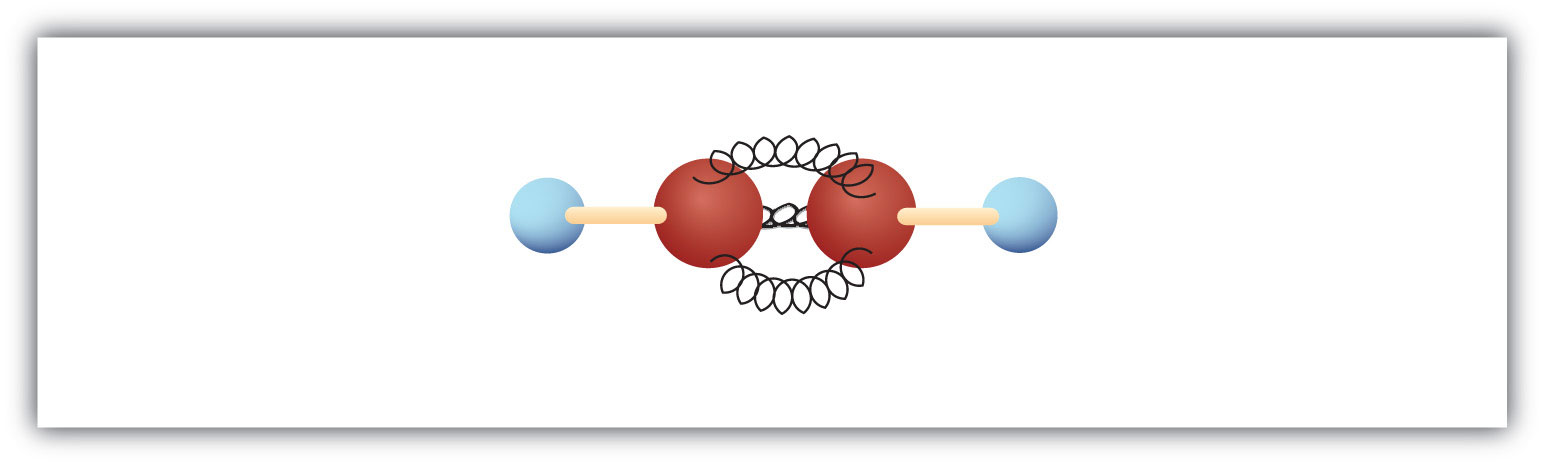
Acetylene (ethyne) is the simplest member of the alkyne family.
Acetylene is used in oxyacetylene torches for cutting and welding metals. The flame from such a torch can be very hot. Most acetylene, however, is converted to chemical intermediates that are used to make vinyl and acrylic plastics, fibers, resins, and a variety of other products.
Alkynes are similar to alkenes in both physical and chemical properties. For example, alkynes undergo many of the typical addition reactions of alkenes. The International Union of Pure and Applied Chemistry (IUPAC) names for alkynes parallel those of alkenes, except that the family ending is -yne rather than -ene. The IUPAC name for acetylene is ethyne. The names of other alkynes are illustrated in the following exercises.
Briefly identify the important differences between an alkene and an alkyne. How are they similar?
The alkene (CH3)2CHCH2CH=CH2 is named 4-methyl-1-pentene. What is the name of (CH3)2CHCH2C≡CH?
Do alkynes show cis-trans isomerism? Explain.
Alkenes have double bonds; alkynes have triple bonds. Both undergo addition reactions.
4-methyl-1-pentyne
No; a triply bonded carbon atom can form only one other bond. It would have to have two groups attached to show cis-trans isomerism.
Draw the structure for each compound.
Draw the structure for each compound.
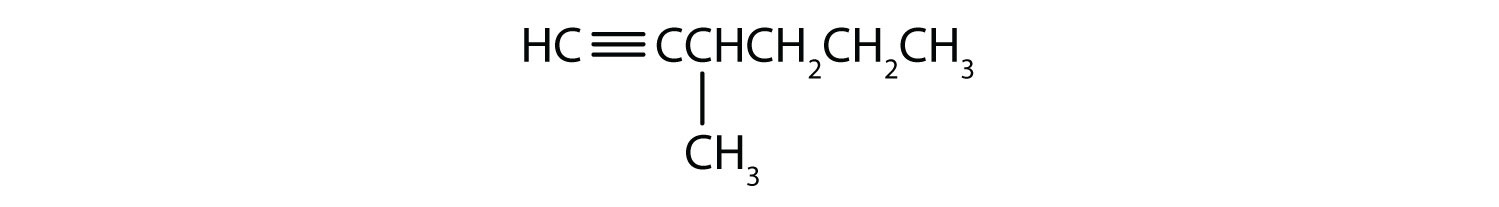
Name each alkyne.