To ensure that you understand the material in this chapter, you should review the meanings of the bold terms in the following summary and ask yourself how they relate to the topics in the chapter.
A protein is a large biological polymer synthesized from amino acids, which are carboxylic acids containing an α-amino group. Proteins have a variety of important roles in living organisms, yet they are made from the same 20 L-amino acids. About half of these amino acids, the essential amino acids, cannot be synthesized by the human body and must be obtained from the diet. In the solid state and in neutral solutions, amino acids exist as zwitterions, species that are charged but electrically neutral. In this form, they behave much like inorganic salts. Each amino acid belongs to one of four classes depending on the characteristics of its R group or amino acid side chain: nonpolar, polar but neutral, positively charged, and negatively charged. Depending on the conditions, amino acids can act as either acids or bases, which means that proteins act as buffers. The pH at which an amino acid exists as the zwitterion is called the isoelectric point (pI).
The amino acids in a protein are linked together by peptide bonds. Protein chains containing 10 or fewer amino acids are usually referred to as peptides, with a prefix such as di- or tri- indicating the number of amino acids. Chains containing more than 50 amino acid units are referred to as proteins or polypeptides. Proteins are classified globular or fibrous, depending on their structure and resulting solubility in water. Globular proteins are nearly spherical and are soluble in water; fibrous proteins have elongated or fibrous structures and are not soluble in water.
Protein molecules can have as many as four levels of structure. The primary structure is the sequence of amino acids in the chain. The secondary structure is the arrangement of adjacent atoms in the peptide chain; the most common arrangements are α-helices or β-pleated sheets. The tertiary structure is the overall three-dimensional shape of the molecule that results from the way the chain bends and folds in on itself. Proteins that consist of more than one chain have quaternary structure, which is the way the multiple chains are packed together.
Four types of intramolecular and intermolecular forces contribute to secondary, tertiary, and quaternary structure: (1) hydrogen bonding between an oxygen or a nitrogen atom and a hydrogen atom bound to an oxygen atom or a nitrogen atom, either on the same chain or on a neighboring chain; (2) ionic bonding between one positively charged side chain and one negatively charged side chain; (3) disulfide linkages between cysteine units; and (4) dispersion forces between nonpolar side chains.
Because of their complexity, protein molecules are delicate and easy to disrupt. A denatured protein is one whose conformation has been changed, in a process called denaturation, so that it can no longer do its physiological job. A variety of conditions, such as heat, ultraviolet radiation, the addition of organic compounds, or changes in pH can denature a protein.
An enzyme is an organic catalyst produced by a living cell. Enzymes are such powerful catalysts that the reactions they promote occur rapidly at body temperature. Without the help of enzymes, these reactions would require high temperatures and long reaction times.
The molecule or molecules on which an enzyme acts are called its substrates. An enzyme has an active site where its substrate or substrates bind to form an enzyme-substrate complex. The reaction occurs, and product is released:
E + S → E–S → E + PThe original lock-and-key model of enzyme and substrate binding pictured a rigid enzyme of unchanging configuration binding to the appropriate substrate. The newer induced-fit model describes the enzyme active site as changing its conformation after binding to the substrate.
Most enzymes have maximal activity in a narrow pH range centered on an optimum pH. In this pH range, the enzyme is correctly folded, and catalytic groups in the active site have the correct charge (positive, negative, or neutral). For most enzymes, the optimum pH is between 6 and 8.
Substances that interfere with enzyme function are called inhibitors. An irreversible inhibitor inactivates enzymes by forming covalent bonds to the enzyme, while a reversible inhibitor inactivates an enzyme by a weaker, noncovalent interaction that is easier to disrupt. A competitive inhibitor is a reversible inhibitor that is structurally similar to the substrate and binds to the active site. When the inhibitor is bound, the substrate is blocked from the active site and no reaction occurs. Because the binding of such an inhibitor is reversible, a high substrate concentration will overcome the inhibition because it increases the likelihood of the substrate binding. A noncompetitive inhibitor binds reversibly at a site distinct from the active site. Thus, it can bind to either the enzyme or the enzyme-substrate complex. The inhibitor changes the conformation of the active site so that the enzyme cannot function properly. Noncompetitive inhibitors are important in feedback inhibition, in which the amount of product produced by a series of reactions is carefully controlled. The final product in a series of reactions acts as a noncompetitive inhibitor of the initial enzyme.
Simple enzymes consist entirely of one or more amino acid chains. Complex enzymes are composed of one or more amino acid chains joined to cofactors—inorganic ions or organic coenzymes. Vitamins are organic compounds that are essential in very small amounts for the maintenance of normal metabolism and generally cannot be synthesized at adequate levels by the body. Vitamins are divided into two broad categories: fat-soluble vitamins and water-soluble vitamins. Many of the water-soluble vitamins are used for the synthesis of coenzymes.
Draw the structure of the amino acid γ-aminobutyric acid (GABA). Would you expect to find GABA in the amino acid sequence of a protein? Explain.
Draw the structure of the amino acid homocysteine (R group = CH2CH2SH). Would you expect to find homocysteine in the amino acid sequence of a protein? Justify your answer.
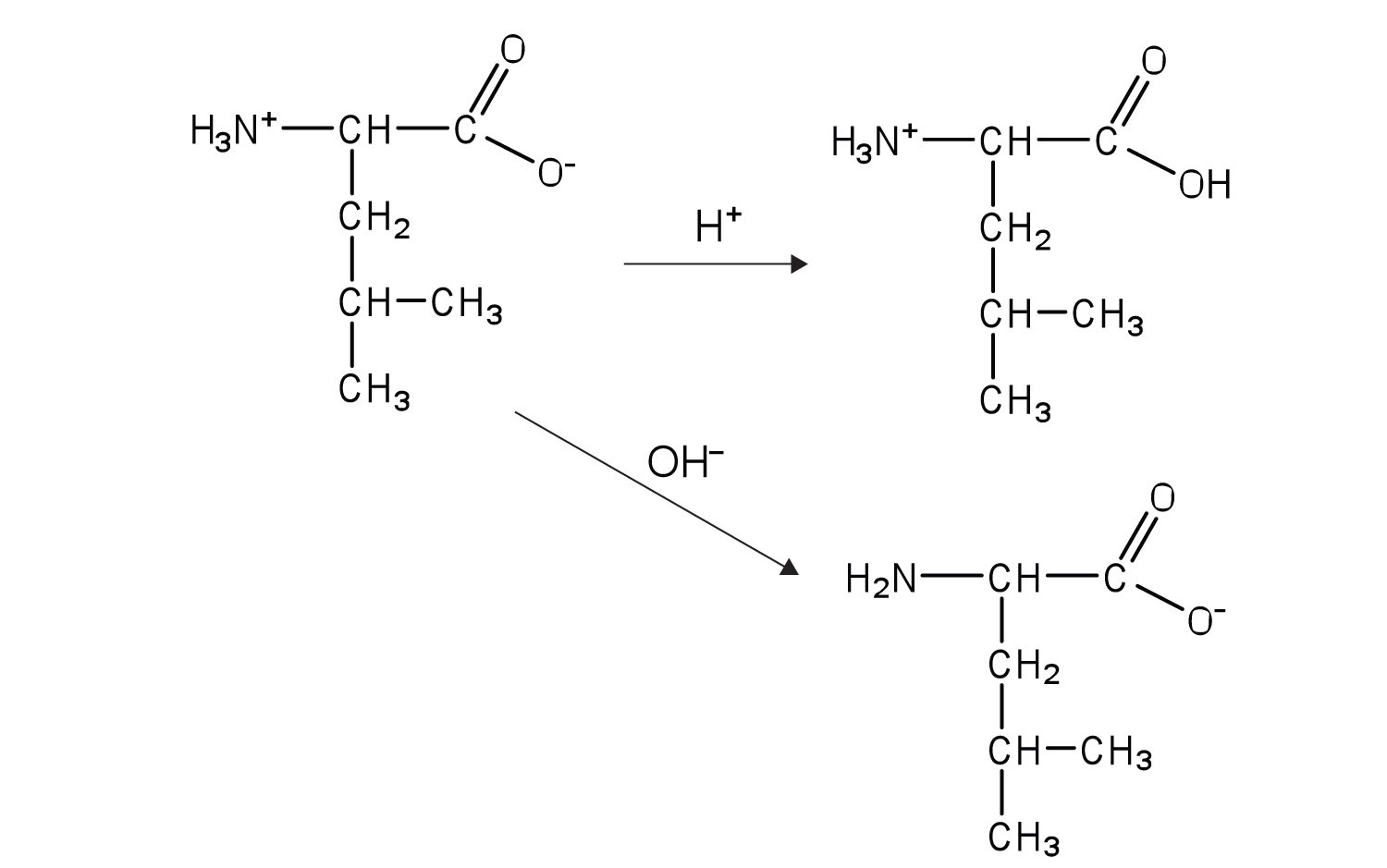
Write equations to show how leucine can act as a buffer (that is, how it can neutralize added acid or base).
Write equations to show how isoleucine can act as a buffer (that is, how it can neutralize added acid or base).
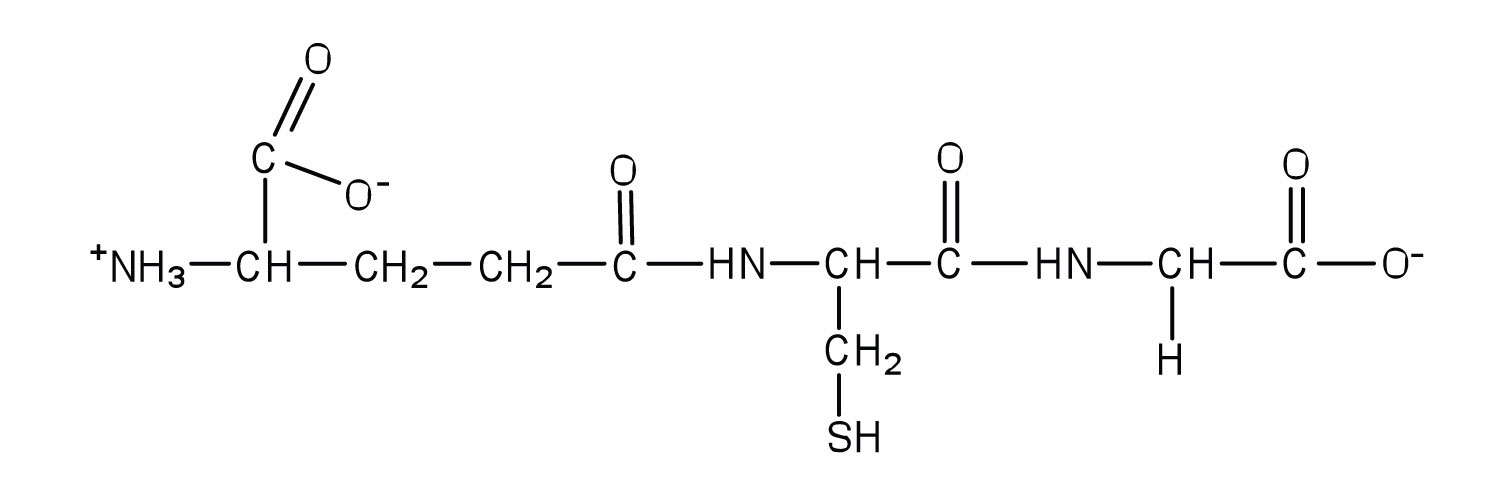
Glutathione (γ-glutamylcysteinylglycine) is a tripeptide found in all cells of higher animals. It contains glutamic acid joined in an unusual peptide linkage involving the carboxyl group of the R group (known as γ-carboxyl group), rather than the usual carboxyl group (the α-carboxyl group). Draw the structure of glutathione.
Draw the structure of the pentapeptide whose sequence is arg-his-gly-leu-asp. Identify which of the amino acids have R groups that can donate or gain hydrogen ions.
Bradykinin is a peptide hormone composed of nine amino acids that lowers blood pressure. Its primary structure is arg-pro-pro-gly-phe-ser-pro-phe-arg. Would you expect bradykinin to be positively charged, negatively charged, or neutral at a pH of 6.0? Justify your answer.
One of the neurotransmitters involved in pain sensation is a peptide called substance P, which is composed of 11 amino acids and is released by nerve-cell terminals in response to pain. Its primary structure is arg-pro-lys-pro-gln-gln-phe-phe-gly-leu-met. Would you expect this peptide to be positively charged, negatively charged, or neutral at a pH of 6.0? Justify your answer.
Carbohydrates are incorporated into glycoproteins. Would you expect the incorporation of sugar units to increase or decrease the solubility of a protein? Justify your answer.
Some proteins have phosphate groups attached through an ester linkage to the OH groups of serine, threonine, or tyrosine residues to form phosphoproteins. Would you expect the incorporation of a phosphate group to increase or decrease the solubility of a protein? Justify your answer.
Refer to Table 18.5 "Classes of Enzymes" and determine how each enzyme would be classified.
Refer to Table 18.5 "Classes of Enzymes" and determine how each enzyme would be classified.
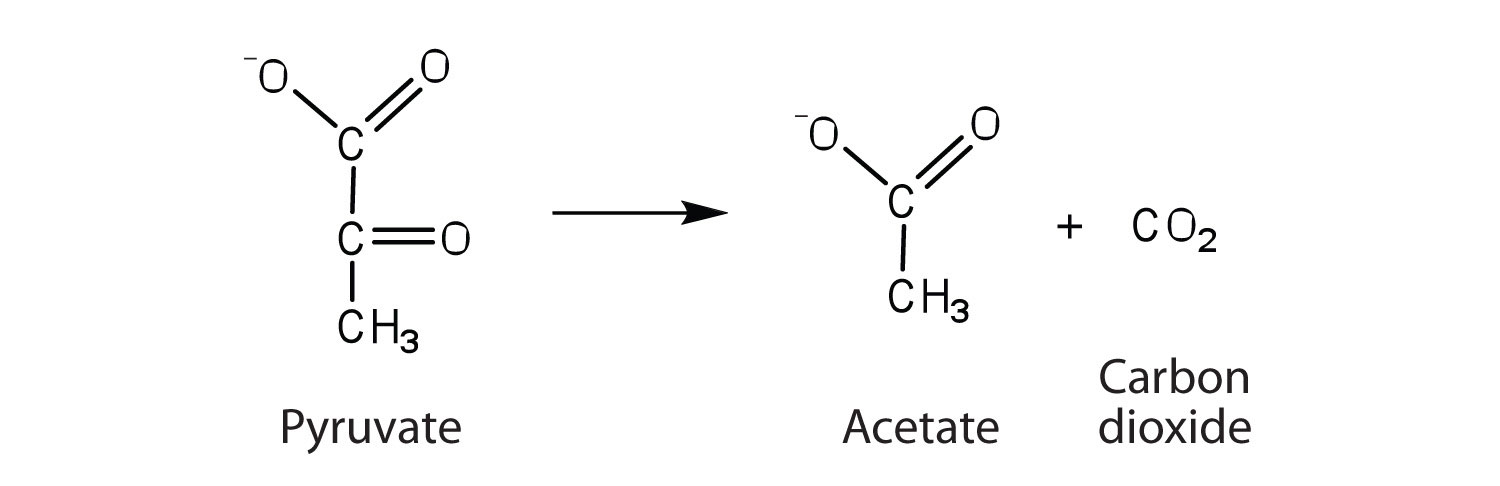
the enzyme that catalyzes the removal of a carboxyl group from pyruvate to form acetate
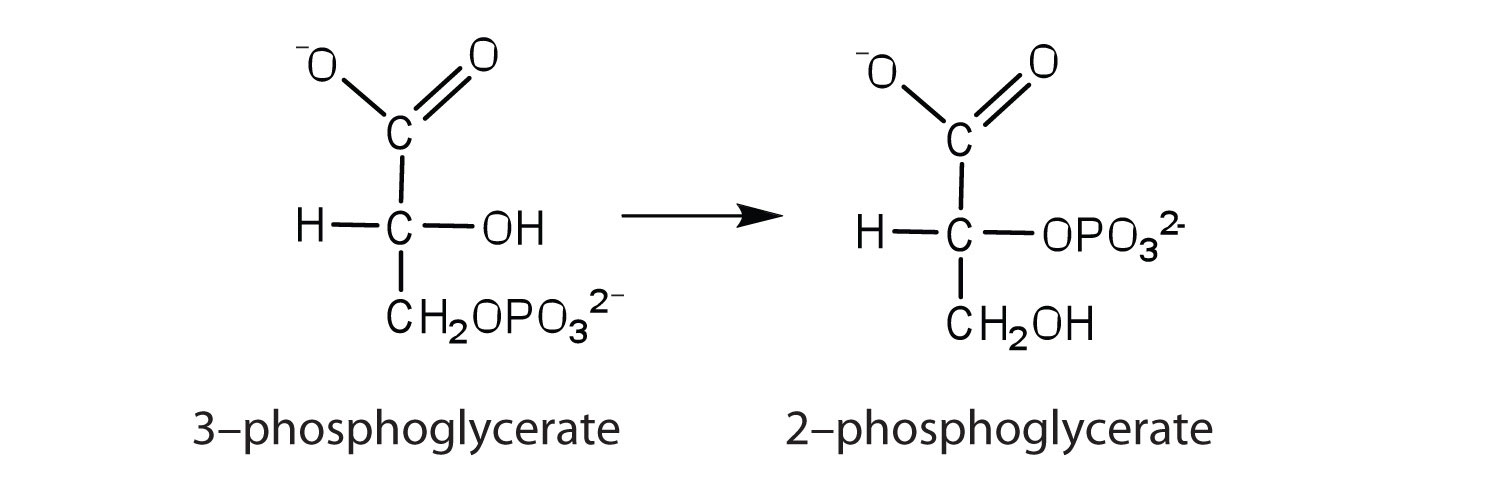
the enzyme that catalyzes the rearrangement of 3-phosphoglycerate to form 2-phosphoglycerate
The enzyme lysozyme has an aspartic acid residue in the active site. In acidic solution, the enzyme is inactive, but activity increases as the pH rises to around 6. Explain why.
The enzyme lysozyme has a glutamic acid residue in the active site. At neutral pH (6–7), the enzyme is active, but activity decreases as the pH rises. Explain why.
The activity of a purified enzyme is measured at a substrate concentration of 1.0 μM and found to convert 49 μmol of substrate to product in 1 min. The activity is measured at 2.0 μM substrate and found to convert 98 μmol of substrate to product/minute.
A patient has a fever of 39°C. Would you expect the activity of enzymes in the body to increase or decrease relative to their activity at normal body temperature (37°C)?
Using your knowledge of factors that influence enzyme activity, describe what happens when milk is pasteurized.
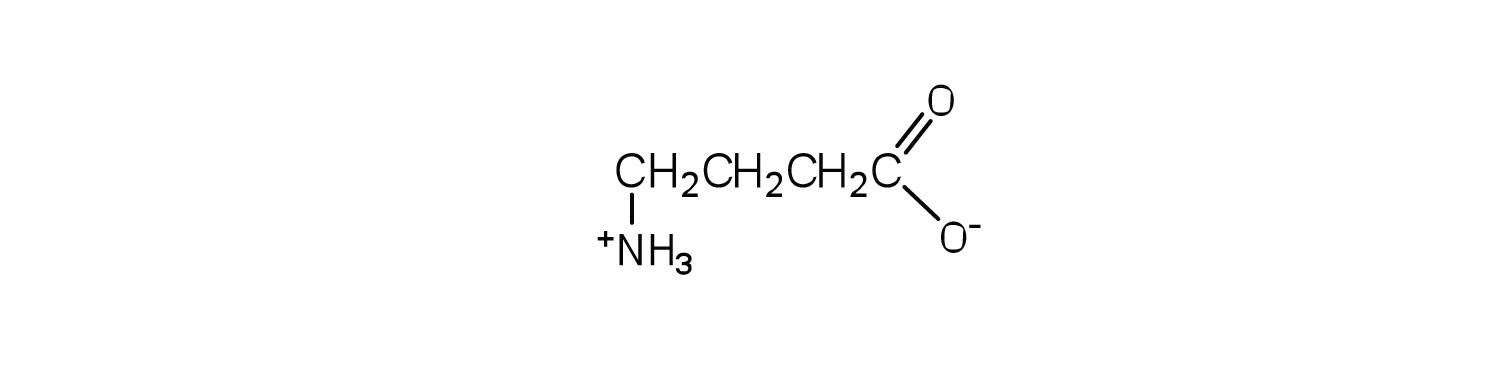
This amino acid would not be found in proteins because it is not an α-amino acid.
Bradykinin would be positively charged; all of the amino acids, except for arginine, have R groups that do not become either positively or negatively charged. The two arginines are R groups that are positively charged at neutral pH, so the peptide would have an overall positive charge.
Carbohydrates have many OH groups attached, which can engage in hydrogen bonding with water, which increases the solubility of the proteins.
The enzyme is active when the carboxyl group in the R group of aspartic acid does not have the hydrogen attached (forming COO–); the hydrogen is removed when the pH of the solution is around pH 6 or higher.
When milk is pasteurized, it is heated to high temperatures. These high temperatures denature the proteins in bacteria, so they cannot carry out needed functions to grow and multiply.