The elements are arranged in a periodic table (Figure 1.24 "The Periodic Table Showing the Elements in Order of Increasing "; also see Chapter 32 "Appendix H: Periodic Table of Elements"), which is probably the single most important learning aid in chemistry. It summarizes huge amounts of information about the elements in a way that permits you to predict many of their properties and chemical reactions. The elements are arranged in seven horizontal rows, in order of increasing atomic number from left to right and top to bottom. The rows are called periodsA row of elements in the periodic table., and they are numbered from 1 to 7. The elements are stacked in such a way that elements with similar chemical properties form vertical columns, called groupsA vertical column of elements in the periodic table. Elements with similar chemical properties reside in the same group., numbered from 1 to 18 (older periodic tables use a system based on roman numerals). Groups 1, 2, and 13–18 are the main group elementsAny element in groups 1, 2, and 13–18 in the periodic table. These groups contain metals, semimetals, and nonmetals., listed as A in older tables. Groups 3–12 are in the middle of the periodic table and are the transition elementsAny element in groups 3–12 in the periodic table. All of the transition elements are metals., listed as B in older tables. The two rows of 14 elements at the bottom of the periodic table are the lanthanides and the actinides, whose positions in the periodic table are indicated in group 3. A more comprehensive description of the periodic table is found in Chapter 7 "The Periodic Table and Periodic Trends".
The heavy orange zigzag line running diagonally from the upper left to the lower right through groups 13–16 in Figure 1.24 "The Periodic Table Showing the Elements in Order of Increasing " divides the elements into metalsAny element to the left of the zigzag line in the periodic table that runs from boron to astatine. All metals except mercury are solids at room temperature and pressure. (in blue, below and to the left of the line) and nonmetalsAny element to the right of the zigzag line in the periodic table that runs from boron to astatine. Nonmetals may be solids, liquids, or gases at room temperature and pressure. (in bronze, above and to the right of the line). As you might expect, elements colored in gold that lie along the diagonal line exhibit properties intermediate between metals and nonmetals; they are called semimetalsAny element that lies adjacent to the zigzag line in the periodic table that runs from boron to astatine. Semimetals (also called metalloids) exhibit properties intermediate between those of metals and nonmetals..
The distinction between metals and nonmetals is one of the most fundamental in chemistry. Metals—such as copper or gold—are good conductors of electricity and heat; they can be pulled into wires because they are ductileThe ability to be pulled into wires. Metals are ductile, whereas nonmetals are usually brittle.; they can be hammered or pressed into thin sheets or foils because they are malleableThe ability to be hammered or pressed into thin sheets or foils. Metals are malleable, whereas nonmetals are usually brittle.; and most have a shiny appearance, so they are lustrousHaving a shiny appearance. Metals are lustrous, whereas nonmetals are not.. The vast majority of the known elements are metals. Of the metals, only mercury is a liquid at room temperature and pressure; all the rest are solids.
Nonmetals, in contrast, are generally poor conductors of heat and electricity and are not lustrous. Nonmetals can be gases (such as chlorine), liquids (such as bromine), or solids (such as iodine) at room temperature and pressure. Most solid nonmetals are brittle, so they break into small pieces when hit with a hammer or pulled into a wire. As expected, semimetals exhibit properties intermediate between metals and nonmetals.
Based on its position in the periodic table, do you expect selenium to be a metal, a nonmetal, or a semimetal?
Given: element
Asked for: classification
Strategy:
Find selenium in the periodic table shown in Figure 1.24 "The Periodic Table Showing the Elements in Order of Increasing " and then classify the element according to its location.
Solution:
The atomic number of selenium is 34, which places it in period 4 and group 16. In Figure 1.24 "The Periodic Table Showing the Elements in Order of Increasing ", selenium lies above and to the right of the diagonal line marking the boundary between metals and nonmetals, so it should be a nonmetal. Note, however, that because selenium is close to the metal-nonmetal dividing line, it would not be surprising if selenium were similar to a semimetal in some of its properties.
Exercise
Based on its location in the periodic table, do you expect indium to be a nonmetal, a metal, or a semimetal?
Answer: metal
As we noted, the periodic table is arranged so that elements with similar chemical behaviors are in the same group. Chemists often make general statements about the properties of the elements in a group using descriptive names with historical origins. For example, the elements of group 1 are known as the alkali metalsAny element in group 1 of the periodic table., group 2 are the alkaline earth metalsAny element in group 2 of the periodic table., group 17 are the halogensDerived from the Greek for “salt forming,” an element in group 17 of the periodic table., and group 18 are the noble gasesAny element in group 18 of the periodic table. All are unreactive monatomic gases at room temperature and pressure..
The alkali metals are lithium, sodium, potassium, rubidium, cesium, and francium. Hydrogen is unique in that it is generally placed in group 1, but it is not a metal.
The compounds of the alkali metals are common in nature and daily life. One example is table salt (sodium chloride); lithium compounds are used in greases, in batteries, and as drugs to treat patients who exhibit manic-depressive, or bipolar, behavior. Although lithium, rubidium, and cesium are relatively rare in nature, and francium is so unstable and highly radioactive that it exists in only trace amounts, sodium and potassium are the seventh and eighth most abundant elements in Earth’s crust, respectively.
The alkaline earth metals are beryllium, magnesium, calcium, strontium, barium, and radium. Beryllium, strontium, and barium are rather rare, and radium is unstable and highly radioactive. In contrast, calcium and magnesium are the fifth and sixth most abundant elements on Earth, respectively; they are found in huge deposits of limestone and other minerals.
The halogens are fluorine, chlorine, bromine, iodine, and astatine. The name halogen is derived from the Greek for “salt forming,” which reflects that all the halogens react readily with metals to form compounds, such as sodium chloride and calcium chloride (used in some areas as road salt).
Compounds that contain the fluoride ion are added to toothpaste and the water supply to prevent dental cavities. Fluorine is also found in Teflon coatings on kitchen utensils. Although chlorofluorocarbon propellants and refrigerants are believed to lead to the depletion of Earth’s ozone layer and contain both fluorine and chlorine, the latter is responsible for the adverse effect on the ozone layer. Bromine and iodine are less abundant than chlorine, and astatine is so radioactive that it exists in only negligible amounts in nature.
The noble gases are helium, neon, argon, krypton, xenon, and radon. Because the noble gases are composed of only single atoms, they are monatomicA species containing a single atom.. At room temperature and pressure, they are unreactive gases. Because of their lack of reactivity, for many years they were called inert gases or rare gases. However, the first chemical compounds containing the noble gases were prepared in 1962. Although the noble gases are relatively minor constituents of the atmosphere, natural gas contains substantial amounts of helium. Because of its low reactivity, argon is often used as an unreactive (inert) atmosphere for welding and in light bulbs. The red light emitted by neon in a gas discharge tube is used in neon lights.
The noble gases are unreactive at room temperature and pressure.
The periodic table is an arrangement of the elements in order of increasing atomic number. Elements that exhibit similar chemistry appear in vertical columns called groups (numbered 1–18 from left to right); the seven horizontal rows are called periods. Some of the groups have widely used common names, including the alkali metals (group 1) and the alkaline earth metals (group 2) on the far left, and the halogens (group 17) and the noble gases (group 18) on the far right. The elements can be broadly divided into metals, nonmetals, and semimetals. Semimetals exhibit properties intermediate between those of metals and nonmetals. Metals are located on the left of the periodic table, and nonmetals are located on the upper right. They are separated by a diagonal band of semimetals. Metals are lustrous, good conductors of electricity, and readily shaped (they are ductile and malleable), whereas solid nonmetals are generally brittle and poor electrical conductors. Other important groupings of elements in the periodic table are the main group elements, the transition metals, the lanthanides, and the actinides.
Classify each element in Conceptual Problem 1 (Section 1.6 "Isotopes and Atomic Masses") as a metal, a nonmetal, or a semimetal. If a metal, state whether it is an alkali metal, an alkaline earth metal, or a transition metal.
Classify each element in Conceptual Problem 2 (Section 1.6 "Isotopes and Atomic Masses") as a metal, a nonmetal, or a semimetal. If a metal, state whether it is an alkali metal, an alkaline earth metal, or a transition metal.
Classify each element as a metal, a semimetal, or a nonmetal. If a metal, state whether it is an alkali metal, an alkaline earth metal, or a transition metal.
Which of these sets of elements are all in the same period?
Which of these sets of elements are all in the same period?
Which of these sets of elements are all in the same group?
Which of these sets of elements are all in the same group?
Indicate whether each element is a transition metal, a halogen, or a noble gas.
Which of the elements indicated in color in the periodic table shown below is most likely to exist as a monoatomic gas? As a diatomic gas? Which is most likely to be a semimetal? A reactive metal?
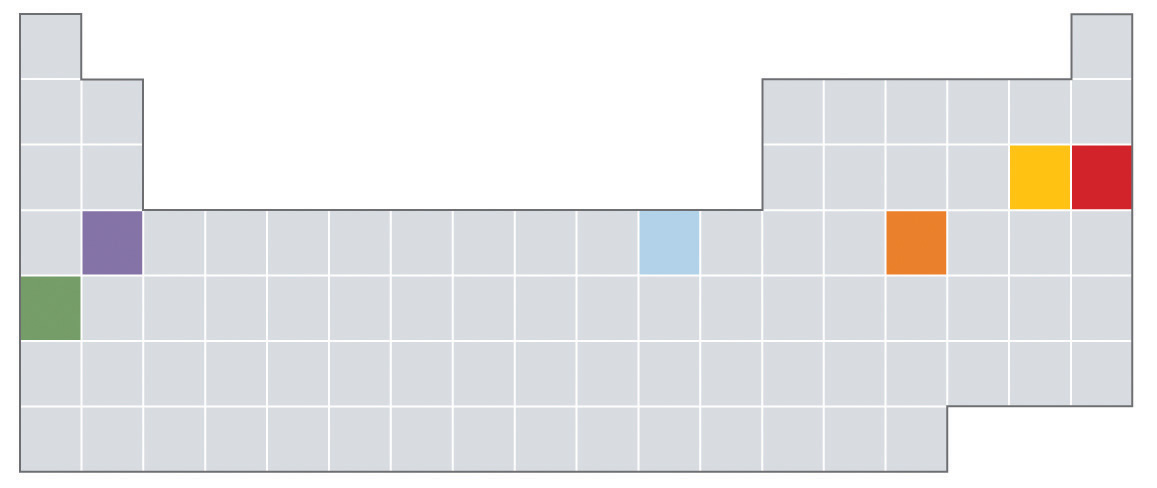
Based on their locations in the periodic table, would you expect these elements to be malleable? Why or why not?
Based on their locations in the periodic table, would you expect these elements to be lustrous? Why or why not?
| Symbol | Type |
|---|---|
| Fe | metal: transition metal |
| Ta | metal: transition metal |
| S | nonmetal |
| Si | semimetal |
| Cl | nonmetal (halogen) |
| Ni | metal: transition metal |
| K | metal: alkali metal |
| Rn | nonmetal (noble gas) |
| Zr | metal: transition metal |