Tungsten bronzes are semimetallic solids that are inert to strong acids and bases, lustrous, and good conductors of electricity; they are used in the production of bronze or metallic paints. These nonstoichiometric compounds have the general formula MxWO3, where M = Na, K, a group 2 metal, or a lanthanide and x < 1. The properties of tungsten bronzes suggest that at least some of the tungsten atoms are in the +6 oxidation state. Given the high oxidation state, why do these solids conduct electricity?
Gout is a painful disorder caused by the overproduction of uric acid, which is deposited as sodium urate crystals in joints. The enzyme that catalyzes the production of uric acid contains Fe3+ and Mo6+. Molecular oxygen is a substrate in this reaction. Based on the oxidation states of the metals, what do you expect one of the products of the reaction to be?
A laboratory technician added aqueous ammonia to an aqueous solution of Mn2+, which produced a pale pink precipitate. She left the solution exposed to air and went home. The next day she returned to the lab and found that her pink precipitate had turned brown-black. Write balanced chemical equations to show what had happened.
Plants use Mn(IV) during photosynthesis to produce dioxygen from water. Write a balanced chemical equation showing this reaction and suggest why Mn is well suited for this purpose.
Rust stains (Fe2O3) can be removed from fabrics by oxalic acid (HO2CCO2H). Write a balanced chemical equation showing the reaction that occurs and predict the solubility of the product in water.
It has been suggested that complexes that can coordinate N2 are used by bacteria to fix atmospheric nitrogen. One such complex is [Ru(NH3)5·N2]Cl2, which was first discovered in 1965. Sigma bonding with N2 in this complex would be weak because the N2 molecule is symmetrical and has zero dipole moment.
Monel metal, which contains 68% Ni, 32% Cu, and traces of Fe and Mn, is highly corrosion resistant. It is used, for example, to make items that will be used in marine environments and to hold corrosive fluorine compounds. Based on its composition, why is Monel particularly suitable for these purposes?
From 1845 to 1850, a fungus known as “potato blight” caused a potato famine in Ireland. Approximately 25% of the Irish population either died or emigrated as a direct result. A spray called Bordeaux mixture is now used to kill the fungus; it is made by the reaction of CuSO4 with Ca(OH)2. What is the formula of the Bordeaux mixture? Write a balanced chemical equation for the reaction.
Many transition metals and their compounds are used as catalysts. Given MnO2, FeCl3, Pt, and Ni, which would you select for each purpose and why?
The Fe2+ site in hemoglobin binds oxygen reversibly; consequently, it is suitable for transporting oxygen in blood. Various other small molecules can bind to the iron instead, thus preventing oxygen transport. Based on the type of bonding, why are CO and NO particularly toxic? Would they be as toxic if hemoglobin contained a V2+ center instead of Fe2+? Why?
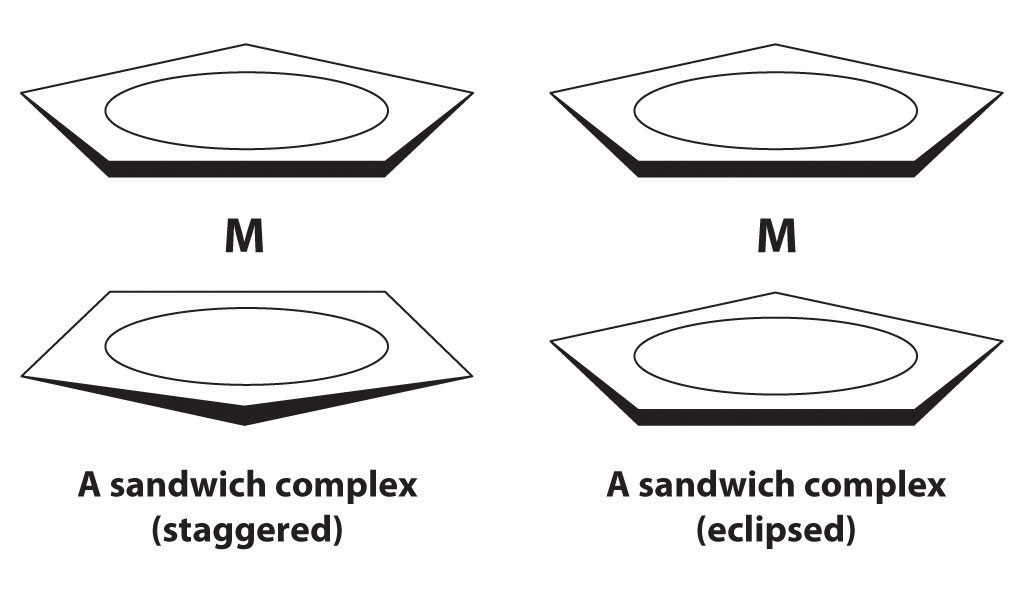
In 1951, G. Wilkinson reported a surprising iron–hydrocarbon compound that is now called ferrocene. Ferrocene is orange and has a structure in which the metal is sandwiched between two planar cyclopentadienyl (C5H5−) rings. It can be viewed as a compound of Fe2+ with two C5H5− rings. Ferrocene does not contain Fe–C σ bonds but another type of bond formed by the lateral overlap of orbitals. Which metal orbitals are involved? A similar structure is obtained with Ru2+. One of these metals forms a sandwich complex that has a staggered conformation, and the other forms a complex that is eclipsed. Which metal produces which conformation? Why?
The Ziegler–Natta catalyst is used for the polymerization of ethylene to form high-density polyethylene, a widely used lightweight plastic. The active form of the catalyst is believed to be TiCl3CH2CH3, and the first step in the polymerization reaction is believed to be binding of the double bond in ethylene to Ti. If you were interested in developing a similar catalyst for this same purpose, would you choose chlorides of Zr, Hf, V, Nb, Ta, Cr, Mo, or W? Why?
Cobalt(II) chloride is used as a visual indicator of humidity because it exists as a blue complex when dry and a pink complex when exposed to moisture in the air. The bonding environment around the cobalt in one of these complexes is octahedral; in the other, it is tetrahedral. What reaction occurs to produce the color change? Write a balanced chemical equation for this reaction, indicating the species present.
A platinum complex that is widely available commercially is chloroplatinic acid {H2[PtCl6]}, which is used to make platinized asbestos, a catalyst. What is the structure of chloroplatinic acid? Is it distorted from an idealized geometry? Do you expect it to be colored? Justify your answers.
The tungsten bronzes can be viewed as the products of partial reduction of WO3 by an active metal to give a mixture of W(VI) and W(V). In the solid, the d orbitals of the metal overlap to form a partially filled band, which is responsible for the luster and metallic conductivity.